当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophilic Triflyl-arylation and Triflyl-pyridylation by Unsymmetrical Aryl/Pyridyl-λ3-iodonium Salts: Synthesis of Aryl and Pyridyl Triflones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.joc.7b01690 Prajwalita Das 1 , Norio Shibata 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.joc.7b01690 Prajwalita Das 1 , Norio Shibata 1
Affiliation
|
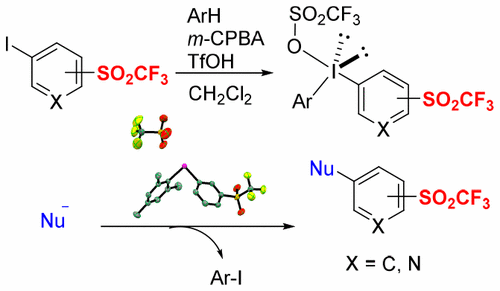
Unsymmetrical diaryl-λ3-iodonium salts containing aryl triflone (Ar-SO2CF3) were designed and synthesized. X-ray crystal structure analysis of the salt indicated a T-shaped geometry at the iodine atom. The salts were found to be powerful electrophilic reagents for triflyl-arylation of C-, N-, and O-centered nucleophiles under mild conditions. Electrophilic transfer of pyridine triflone (Py-SO2CF3) to nucleophiles was also realized by the use of corresponding triflylpyridyl-aryl-λ3-iodonium salts. Selection of auxiliaries (dummy ligands) of unsymmetrical diaryl-λ3-iodonium salts is crucial for this transformation.
中文翻译:

电三氟甲磺酰基芳基化和三氟甲磺酰基- pyridylation通过不对称芳基/吡啶基λ 3 -iodonium盐:芳基和吡啶基Triflones的合成
非对称二芳基- λ 3 -iodonium含有芳triflone(AR-SO盐2 CF 3),设计并合成。盐的X射线晶体结构分析表明在碘原子处为T形。发现该盐是在温和条件下用于以C-,N-和O-为中心的亲核试剂三氟芳基化的强力亲电试剂。吡啶triflone的电子转移(PY-SO 2 CF 3),以亲核试剂也通过使用对应triflylpyridyl芳基λ的实现3 -iodonium盐。不对称的二芳基-λ的助剂(虚设的配体)的选定3-碘盐对于该转化至关重要。
更新日期:2017-09-07
中文翻译:

电三氟甲磺酰基芳基化和三氟甲磺酰基- pyridylation通过不对称芳基/吡啶基λ 3 -iodonium盐:芳基和吡啶基Triflones的合成
非对称二芳基- λ 3 -iodonium含有芳triflone(AR-SO盐2 CF 3),设计并合成。盐的X射线晶体结构分析表明在碘原子处为T形。发现该盐是在温和条件下用于以C-,N-和O-为中心的亲核试剂三氟芳基化的强力亲电试剂。吡啶triflone的电子转移(PY-SO 2 CF 3),以亲核试剂也通过使用对应triflylpyridyl芳基λ的实现3 -iodonium盐。不对称的二芳基-λ的助剂(虚设的配体)的选定3-碘盐对于该转化至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号