Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrophilicity of Nonanoic Acid and Its Conjugate Base at the Air/Water Interface
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acsomega.7b00960 Shiva Badban 1 , Anita E Hyde 1 , Chi M Phan 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acsomega.7b00960 Shiva Badban 1 , Anita E Hyde 1 , Chi M Phan 1
Affiliation
|
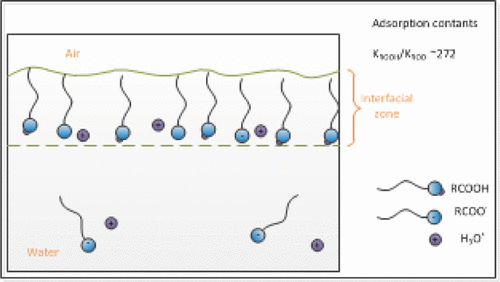
A general adsorption model based on partial dissociation was developed for carboxylic acids. The model was applied to the adsorption of nonanoic acid at the air/water interface. Two cases were selected for experimental verification: acid-only and acid with a constant Na+OH– concentration. The model was applied simultaneously at both conditions, and the hydrophilicity of the ionic states was quantified by the adsorption constants, KA and KAH. It was found that the adsorption constant for the acidic group is significantly higher than that for the carboxylate group, KAH/KA ∼ 272. The model lays important groundwork for modeling and predicting carboxylic/carboxylate adsorption.
中文翻译:

壬酸及其共轭碱在空气/水界面的亲水性
针对羧酸开发了基于部分解离的通用吸附模型。该模型应用于壬酸在空气/水界面的吸附。选择两种情况进行实验验证:仅酸和具有恒定Na + OH-浓度的酸。该模型在两种条件下同时应用,离子态的亲水性通过吸附常数KA和K AH进行量化。结果发现,酸性基团的吸附常数明显高于羧酸盐基团的吸附常数,K AH / KA ∼ 272。该模型为建模和预测羧酸/羧酸盐吸附奠定了重要基础。
更新日期:2017-09-07
中文翻译:

壬酸及其共轭碱在空气/水界面的亲水性
针对羧酸开发了基于部分解离的通用吸附模型。该模型应用于壬酸在空气/水界面的吸附。选择两种情况进行实验验证:仅酸和具有恒定Na + OH-浓度的酸。该模型在两种条件下同时应用,离子态的亲水性通过吸附常数KA和K AH进行量化。结果发现,酸性基团的吸附常数明显高于羧酸盐基团的吸附常数,K AH / KA ∼ 272。该模型为建模和预测羧酸/羧酸盐吸附奠定了重要基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号