当前位置:
X-MOL 学术
›
JAMA Pediatr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Association of Serum Soluble Urokinase Receptor Levels With Progression of Kidney Disease in Children
JAMA Pediatrics ( IF 24.7 ) Pub Date : 2017-11-06 , DOI: 10.1001/jamapediatrics.2017.2914 Franz Schaefer 1 , Howard Trachtman 2 , Elke Wühl 1 , Marietta Kirchner 3 , Salim S Hayek 4 , Ali Anarat 5 , Ali Duzova 6 , Sevgi Mir 7 , Dusan Paripovic 8 , Alev Yilmaz 9 , Francesca Lugani 10 , Klaus Arbeiter 11 , Mieczyslaw Litwin 12 , Jun Oh 13 , Maria Chiara Matteucci 14 , Jutta Gellermann 15 , Simone Wygoda 16 , Augustina Jankauskiene 17 , Günter Klaus 18 , Jiri Dusek 19 , Sara Testa 20 , Aleksandra Zurowska 21 , Alberto Caldas Afonso 22 , Melissa Tracy 23 , Changli Wei 23 , Sanja Sever 24 , William Smoyer 25 , Jochen Reiser 23 ,
JAMA Pediatrics ( IF 24.7 ) Pub Date : 2017-11-06 , DOI: 10.1001/jamapediatrics.2017.2914 Franz Schaefer 1 , Howard Trachtman 2 , Elke Wühl 1 , Marietta Kirchner 3 , Salim S Hayek 4 , Ali Anarat 5 , Ali Duzova 6 , Sevgi Mir 7 , Dusan Paripovic 8 , Alev Yilmaz 9 , Francesca Lugani 10 , Klaus Arbeiter 11 , Mieczyslaw Litwin 12 , Jun Oh 13 , Maria Chiara Matteucci 14 , Jutta Gellermann 15 , Simone Wygoda 16 , Augustina Jankauskiene 17 , Günter Klaus 18 , Jiri Dusek 19 , Sara Testa 20 , Aleksandra Zurowska 21 , Alberto Caldas Afonso 22 , Melissa Tracy 23 , Changli Wei 23 , Sanja Sever 24 , William Smoyer 25 , Jochen Reiser 23 ,
Affiliation
|
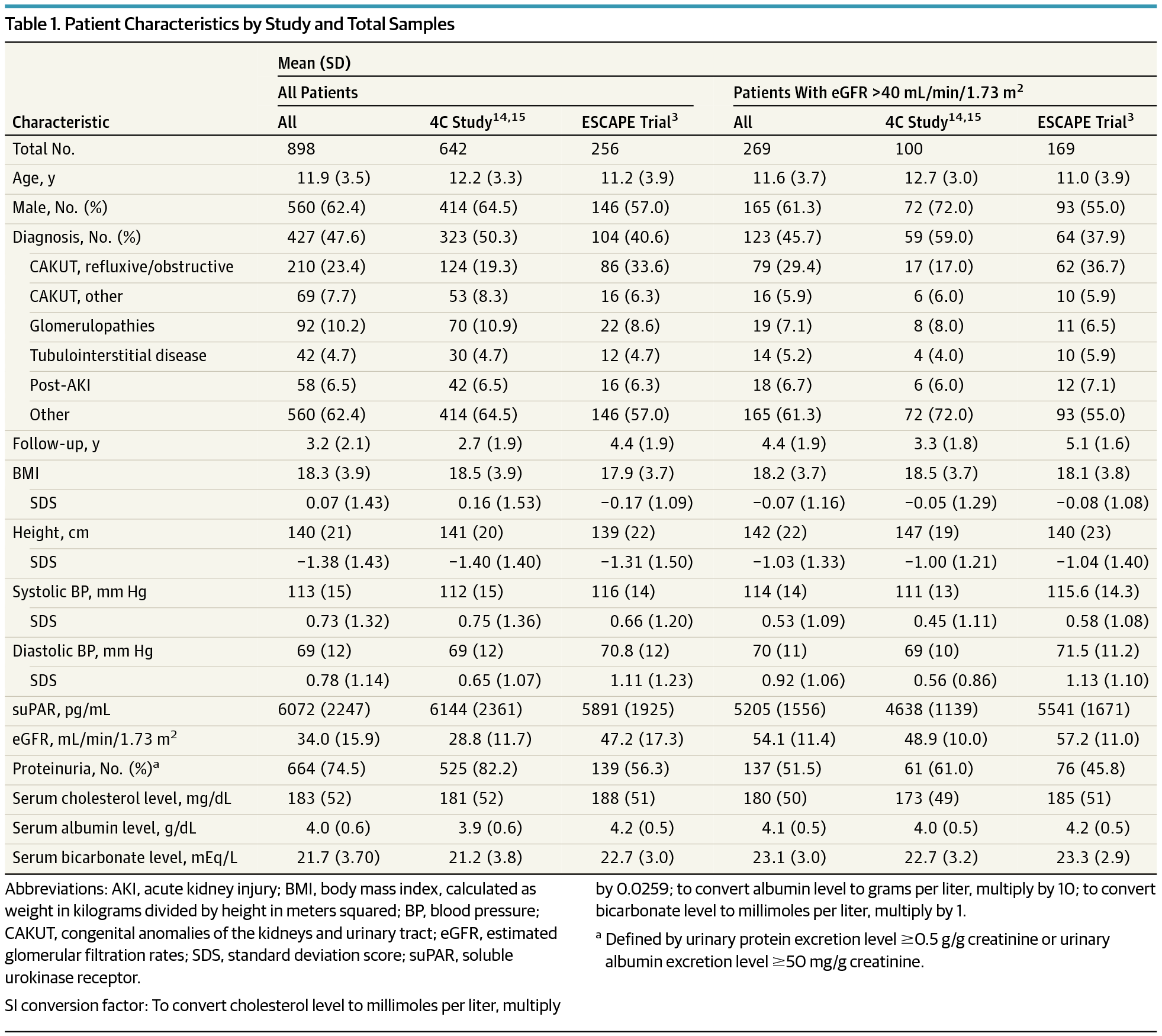
Importance Conventional methods to diagnose and monitor chronic kidney disease (CKD) in children, such as creatinine level and cystatin C–derived estimated glomerular filtration rate (eGFR) and assessment of proteinuria in spot or timed urine samples, are of limited value in identifying patients at risk of progressive kidney function loss. Serum soluble urokinase receptor (suPAR) levels strongly predict incident CKD stage 3 in adults. Objective To determine whether elevated suPAR levels are associated with renal disease progression in children with CKD. Design, Setting, and Participants Post hoc analysis of 2 prospectively followed up pediatric CKD cohorts, ie, the ESCAPE Trial (1999-2007) and the 4C Study (2010-2016), with serum suPAR level measured at enrollment and longitudinal eGFR measured prospectively. In the 2 trials, a total of 898 children were observed at 30 (ESCAPE Trial; n = 256) and 55 (4C Study; n = 642) tertiary care hospitals in 13 European countries. Renal diagnoses included congenital anomalies of the kidneys and urinary tract (n = 637 [70.9%]), tubulointerstitial nephropathies (n = 92 [10.2%]), glomerulopathies (n = 69 [7.7%]), postischemic CKD (n = 42 [4.7%]), and other CKD (n = 58 [6.5%]). Total follow-up duration was up to 7.9 years, and median follow-up was 3.1 years. Analyses were conducted from October 2016 to December 2016. Exposures Serum suPAR level was measured at enrollment, and eGFR was measured every 2 months in the ESCAPE Trial and every 6 months in the 4C Study. The primary end point of CKD progression was a composite of 50% eGFR loss, eGFR less than 10 mL/min/1.73 m2, or initiation of renal replacement therapy. Main Outcomes and Measures The primary end point in this study was renal survival, defined as a composite of 50% loss of GFR that persisted for at least 1 month, the start of renal replacement therapy, or an eGFR less than 10 mL/min/1.73 m2. Results Of the 898 included children, 560 (62.4%) were male, and the mean (SD) patient age at enrollment was 11.9 (3.5) years. The mean (SD) eGFR was 34 (16) mL/min/1.73 m2. The 5-year end point–free renal survival was 64.5% (95% CI, 57.4-71.7) in children with suPAR levels in the lowest quartile compared with 35.9% (95% CI, 28.7-43.0) in those in the highest quartile (P < .001). By multivariable analysis, the risk of attaining the end point was higher in children with glomerulopathies and increased with age, blood pressure, proteinuria, and lower eGFR at baseline. In patients with baseline eGFR greater than 40 mL/min/1.73 m2, higher log-transformed suPAR levels were associated with a higher risk of CKD progression after adjustment for traditional risk factors (hazard ratio, 5.12; 95% CI, 1.56-16.7; P = .007). Conclusions and Relevance Patients with high suPAR levels were more likely to have progression of their kidney disease. Further studies should determine whether suPAR levels can identify children at risk for future CKD.
中文翻译:

血清可溶性尿激酶受体水平与儿童肾脏疾病进展的关系
诊断和监测儿童慢性肾病 (CKD) 的传统方法,例如肌酐水平和胱抑素 C 衍生的估计肾小球滤过率 (eGFR) 以及点检或定时尿样中的蛋白尿评估,在识别患者方面价值有限存在进行性肾功能丧失的风险。血清可溶性尿激酶受体 (suPAR) 水平强烈预测成人 CKD 3 期的发生。目的 确定 suPAR 水平升高是否与 CKD 儿童肾脏疾病进展相关。设计、设置和参与者 对 2 个前瞻性随访儿科 CKD 队列(即 ESCAPE 试验(1999-2007)和 4C 研究(2010-2016))进行事后分析,在入组时测量血清 suPAR 水平,并前瞻性测量纵向 eGFR 。在这 2 项试验中,共有 898 名儿童在 13 个欧洲国家的 30 家(ESCAPE 试验;n = 256)和 55 家(4C 研究;n = 642)三级护理医院进行了观察。肾脏诊断包括肾脏和泌尿道先天性异常 (n = 637 [70.9%])、肾小管间质性肾病 (n = 92 [10.2%])、肾小球病 (n = 69 [7.7%])、缺血后 CKD (n = 42) [4.7%])和其他 CKD(n = 58 [6.5%])。总随访时间长达 7.9 年,中位随访时间为 3.1 年。分析于 2016 年 10 月至 2016 年 12 月进行。暴露 血清 suPAR 水平在入组时测量,eGFR 在 ESCAPE 试验中每 2 个月测量一次,在 4C 研究中每 6 个月测量一次 eGFR。 CKD 进展的主要终点是 eGFR 损失 50%、eGFR 低于 10 mL/min/1.73 m2 或开始肾脏替代治疗的复合终点。 主要结果和措施 本研究的主要终点是肾脏存活率,定义为持续至少 1 个月的 GFR 下降 50%、开始肾脏替代治疗或 eGFR 低于 10 mL/min/ 1.73 平方米。结果 在 898 名儿童中,560 名 (62.4%) 为男性,入组时患者平均 (SD) 年龄为 11.9 (3.5) 岁。平均 (SD) eGFR 为 34 (16) mL/min/1.73 m2。 suPAR 水平处于最低四分位数的儿童的 5 年无终点肾生存率为 64.5%(95% CI,57.4-71.7),而处于最高四分位数的儿童的 5 年无终点肾生存率为 35.9%(95% CI,28.7-43.0) (P < .001)。通过多变量分析,患有肾小球病的儿童达到终点的风险较高,并且随着年龄、血压、蛋白尿和基线 eGFR 较低而增加。在基线 eGFR 大于 40 mL/min/1.73 m2 的患者中,调整传统危险因素后,较高的对数转换 suPAR 水平与较高的 CKD 进展风险相关(风险比,5.12;95% CI,1.56-16.7; P = .007)。结论和相关性 suPAR 水平高的患者肾脏疾病进展的可能性更大。进一步的研究应该确定 suPAR 水平是否可以识别未来有 CKD 风险的儿童。
更新日期:2017-11-06
中文翻译:

血清可溶性尿激酶受体水平与儿童肾脏疾病进展的关系
诊断和监测儿童慢性肾病 (CKD) 的传统方法,例如肌酐水平和胱抑素 C 衍生的估计肾小球滤过率 (eGFR) 以及点检或定时尿样中的蛋白尿评估,在识别患者方面价值有限存在进行性肾功能丧失的风险。血清可溶性尿激酶受体 (suPAR) 水平强烈预测成人 CKD 3 期的发生。目的 确定 suPAR 水平升高是否与 CKD 儿童肾脏疾病进展相关。设计、设置和参与者 对 2 个前瞻性随访儿科 CKD 队列(即 ESCAPE 试验(1999-2007)和 4C 研究(2010-2016))进行事后分析,在入组时测量血清 suPAR 水平,并前瞻性测量纵向 eGFR 。在这 2 项试验中,共有 898 名儿童在 13 个欧洲国家的 30 家(ESCAPE 试验;n = 256)和 55 家(4C 研究;n = 642)三级护理医院进行了观察。肾脏诊断包括肾脏和泌尿道先天性异常 (n = 637 [70.9%])、肾小管间质性肾病 (n = 92 [10.2%])、肾小球病 (n = 69 [7.7%])、缺血后 CKD (n = 42) [4.7%])和其他 CKD(n = 58 [6.5%])。总随访时间长达 7.9 年,中位随访时间为 3.1 年。分析于 2016 年 10 月至 2016 年 12 月进行。暴露 血清 suPAR 水平在入组时测量,eGFR 在 ESCAPE 试验中每 2 个月测量一次,在 4C 研究中每 6 个月测量一次 eGFR。 CKD 进展的主要终点是 eGFR 损失 50%、eGFR 低于 10 mL/min/1.73 m2 或开始肾脏替代治疗的复合终点。 主要结果和措施 本研究的主要终点是肾脏存活率,定义为持续至少 1 个月的 GFR 下降 50%、开始肾脏替代治疗或 eGFR 低于 10 mL/min/ 1.73 平方米。结果 在 898 名儿童中,560 名 (62.4%) 为男性,入组时患者平均 (SD) 年龄为 11.9 (3.5) 岁。平均 (SD) eGFR 为 34 (16) mL/min/1.73 m2。 suPAR 水平处于最低四分位数的儿童的 5 年无终点肾生存率为 64.5%(95% CI,57.4-71.7),而处于最高四分位数的儿童的 5 年无终点肾生存率为 35.9%(95% CI,28.7-43.0) (P < .001)。通过多变量分析,患有肾小球病的儿童达到终点的风险较高,并且随着年龄、血压、蛋白尿和基线 eGFR 较低而增加。在基线 eGFR 大于 40 mL/min/1.73 m2 的患者中,调整传统危险因素后,较高的对数转换 suPAR 水平与较高的 CKD 进展风险相关(风险比,5.12;95% CI,1.56-16.7; P = .007)。结论和相关性 suPAR 水平高的患者肾脏疾病进展的可能性更大。进一步的研究应该确定 suPAR 水平是否可以识别未来有 CKD 风险的儿童。











































 京公网安备 11010802027423号
京公网安备 11010802027423号