当前位置:
X-MOL 学术
›
N. Engl. J. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tiotropium in Early-Stage Chronic Obstructive Pulmonary Disease.
The New England Journal of Medicine ( IF 158.5 ) Pub Date : 2017-09-07 , DOI: 10.1056/nejmoa1700228 Yumin Zhou 1 , Nan-Shan Zhong 1 , Xiaochen Li 1 , Shuyun Chen 1 , Jinping Zheng 1 , Dongxing Zhao 1 , Weimin Yao 1 , Rongchang Zhi 1 , Liping Wei 1 , Bingwen He 1 , Xiangyan Zhang 1 , Changli Yang 1 , Ying Li 1 , Fenglei Li 1 , Juan Du 1 , Jianping Gui 1 , Bin Hu 1 , Chunxue Bai 1 , Ping Huang 1 , Gang Chen 1 , Yongjian Xu 1 , Changzheng Wang 1 , Biao Liang 1 , Yinhuan Li 1 , Guoping Hu 1 , Hui Tan 1 , Xianwei Ye 1 , Xitao Ma 1 , Yan Chen 1 , Xiwei Hu 1 , Jia Tian 1 , Xiaodan Zhu 1 , Zhe Shi 1 , Xiufang Du 1 , Minjing Li 1 , Shengming Liu 1 , Ronghuan Yu 1 , Jianping Zhao 1 , Qianli Ma 1 , Canmao Xie 1 , Xiongbin Li 1 , Tao Chen 1 , Yingxiang Lin 1 , Lizhen Zeng 1 , Changxiu Ye 1 , Weishu Ye 1 , Xiangwen Luo 1 , Lingshan Zeng 1 , Shuqing Yu 1 , Wei-Jie Guan 1 , Pixin Ran 1
The New England Journal of Medicine ( IF 158.5 ) Pub Date : 2017-09-07 , DOI: 10.1056/nejmoa1700228 Yumin Zhou 1 , Nan-Shan Zhong 1 , Xiaochen Li 1 , Shuyun Chen 1 , Jinping Zheng 1 , Dongxing Zhao 1 , Weimin Yao 1 , Rongchang Zhi 1 , Liping Wei 1 , Bingwen He 1 , Xiangyan Zhang 1 , Changli Yang 1 , Ying Li 1 , Fenglei Li 1 , Juan Du 1 , Jianping Gui 1 , Bin Hu 1 , Chunxue Bai 1 , Ping Huang 1 , Gang Chen 1 , Yongjian Xu 1 , Changzheng Wang 1 , Biao Liang 1 , Yinhuan Li 1 , Guoping Hu 1 , Hui Tan 1 , Xianwei Ye 1 , Xitao Ma 1 , Yan Chen 1 , Xiwei Hu 1 , Jia Tian 1 , Xiaodan Zhu 1 , Zhe Shi 1 , Xiufang Du 1 , Minjing Li 1 , Shengming Liu 1 , Ronghuan Yu 1 , Jianping Zhao 1 , Qianli Ma 1 , Canmao Xie 1 , Xiongbin Li 1 , Tao Chen 1 , Yingxiang Lin 1 , Lizhen Zeng 1 , Changxiu Ye 1 , Weishu Ye 1 , Xiangwen Luo 1 , Lingshan Zeng 1 , Shuqing Yu 1 , Wei-Jie Guan 1 , Pixin Ran 1
Affiliation
|
BACKGROUND
Patients with mild or moderate chronic obstructive pulmonary disease (COPD) rarely receive medications, because they have few symptoms. We hypothesized that long-term use of tiotropium would improve lung function and ameliorate the decline in lung function in patients with mild or moderate COPD.
METHODS
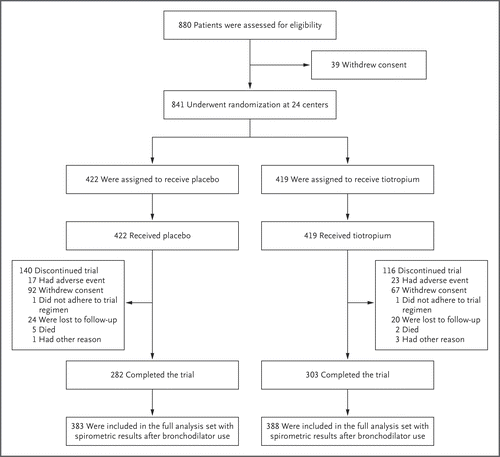
In a multicenter, randomized, double-blind, placebo-controlled trial that was conducted in China, we randomly assigned 841 patients with COPD of Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1 (mild) or 2 (moderate) severity to receive a once-daily inhaled dose (18 μg) of tiotropium (419 patients) or matching placebo (422) for 2 years. The primary end point was the between-group difference in the change from baseline to 24 months in the forced expiratory volume in 1 second (FEV1) before bronchodilator use. Secondary end points included the between-group difference in the change from baseline to 24 months in the FEV1 after bronchodilator use and the between-group difference in the annual decline in the FEV1 before and after bronchodilator use from day 30 to month 24.
RESULTS
Of 841 patients who underwent randomization, 388 patients in the tiotropium group and 383 in the placebo group were included in the full analysis set. The FEV1 in patients who received tiotropium was higher than in those who received placebo throughout the trial (ranges of mean differences, 127 to 169 ml before bronchodilator use and 71 to 133 ml after bronchodilator use; P<0.001 for all comparisons). There was no significant amelioration of the mean (±SE) annual decline in the FEV1 before bronchodilator use: the decline was 38±6 ml per year in the tiotropium group and 53±6 ml per year in the placebo group (difference, 15 ml per year; 95% confidence interval [CI], -1 to 31; P=0.06). In contrast, the annual decline in the FEV1 after bronchodilator use was significantly less in the tiotropium group than in the placebo group (29±5 ml per year vs. 51±6 ml per year; difference, 22 ml per year [95% CI, 6 to 37]; P=0.006). The incidence of adverse events was generally similar in the two groups.
CONCLUSIONS
Tiotropium resulted in a higher FEV1 than placebo at 24 months and ameliorated the annual decline in the FEV1 after bronchodilator use in patients with COPD of GOLD stage 1 or 2. (Funded by Boehringer Ingelheim and others; Tie-COPD ClinicalTrials.gov number, NCT01455129 .).
更新日期:2017-09-07



























 京公网安备 11010802027423号
京公网安备 11010802027423号