JAMA Psychiatry ( IF 25.8 ) Pub Date : 2017-09-01 , DOI: 10.1001/jamapsychiatry.2017.1838 Kelly E. Dunn 1 , D. Andrew Tompkins 1 , George E. Bigelow 1 , Eric C. Strain 1
|
Importance Opioid use disorder (OUD) is a significant public health problem. Supervised withdrawal (ie, detoxification) from opioids using clonidine or buprenorphine hydrochloride is a widely used treatment.
Objective To evaluate whether tramadol hydrochloride extended-release (ER), an approved analgesic with opioid and nonopioid mechanisms of action and low abuse potential, is effective for use in supervised withdrawal settings.
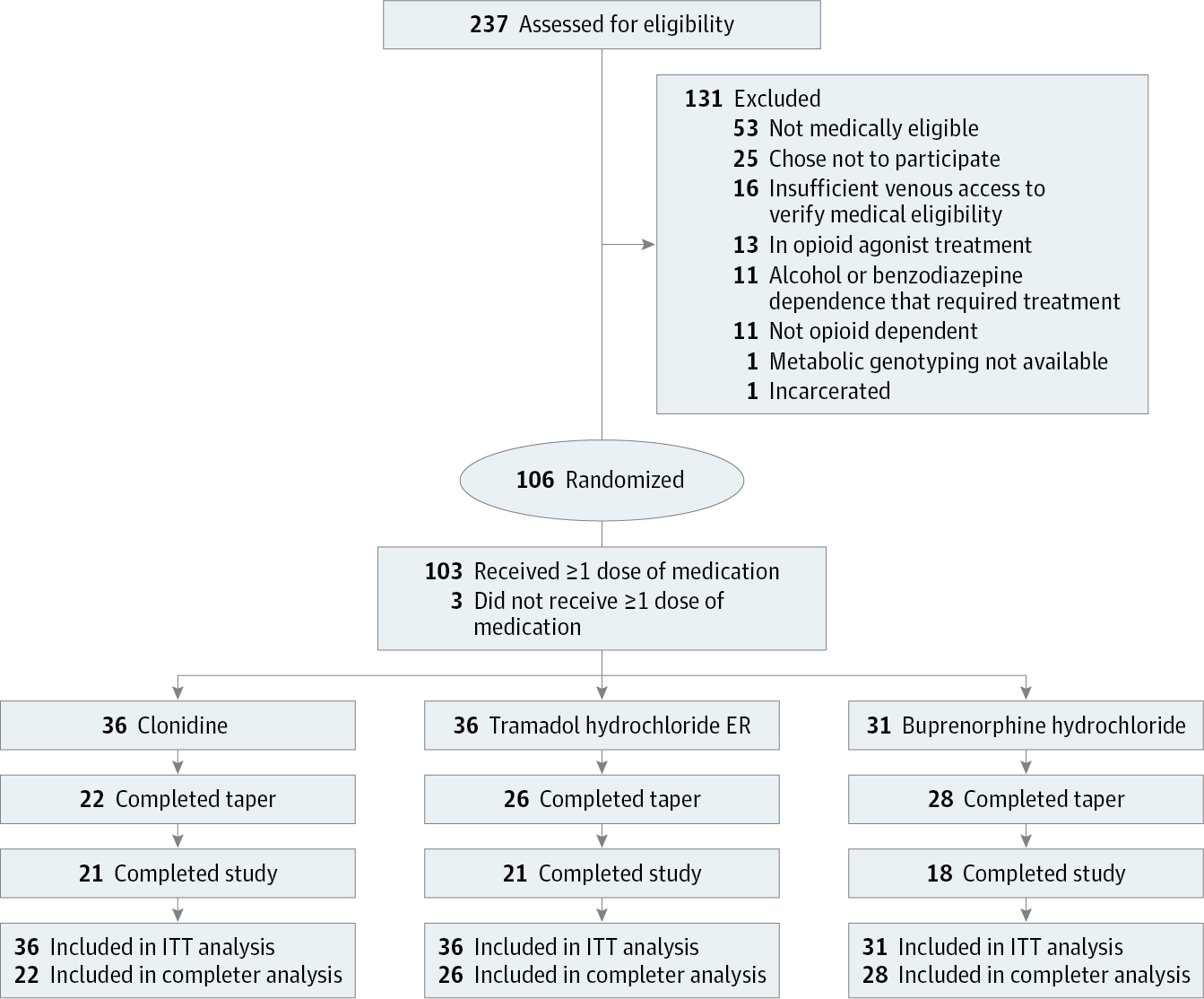
Design, Setting, and Participants A randomized clinical trial was conducted in a residential research setting with 103 participants with OUD. Participants’ treatment was stabilized with morphine, 30 mg, administered subcutaneously 4 times daily. A 7-day taper using clonidine (n = 36), tramadol ER (n = 36), or buprenorphine (n = 31) was then instituted, and patients were crossed-over to double-blind placebo during a post-taper period. The study was conducted from October 25, 2010, to June 23, 2015.
Main Outcomes and Measures Retention, withdrawal symptom management, concomitant medication utilization, and naltrexone induction. Results were analyzed over time and using area under the curve for the intention-to-treat and completer groups.
Results Of the 103 participants, 88 (85.4%) were men and 43 (41.7%) were white; mean (SD) age was 28.9 (10.4) years. Buprenorphine participants (28 [90.3%]) were significantly more likely to be retained at the end of the taper compared with clonidine participants (22 [61.1%]); tramadol ER retention was intermediate and did not differ significantly from that of the other groups (26 [72.2%]; χ2 = 8.5, P = .01). Time-course analyses of withdrawal revealed significant effects of phase (taper, post taper) for the Clinical Opiate Withdrawal Scale (COWS) score (taper mean, 5.19 [SE, .26]; post-taper mean, 3.97 [SE, .23]; F2,170 = 3.6, P = .03) and Subjective Opiate Withdrawal Scale (SOWS) score (taper mean,8.81 [SE, .40]; post-taper mean, 4.14 [SE, .30]; F2,170 = 15.7, P < .001), but no group effects or group × phase interactions. Analyses of area under the curve of SOWS total scores showed significant reductions (F2,159 = 17.7, P < .001) in withdrawal severity between the taper and post-taper periods for clonidine (taper mean, 13.1; post-taper mean, 3.2; P < .001) and tramadol ER (taper mean, 7.4; post-taper mean, 2.8; P = .03), but not buprenorphine (taper mean, 6.4; post-taper mean, 7.4). Use of concomitant medication increased significantly (F2,159 = 30.7, P < .001) from stabilization to taper in the clonidine (stabilization mean, 0.64 [SE, .05]; taper mean, 1.54 [SE, .10]; P < .001) and tramadol ER (stabilization mean, 0.53 [SE, .05]; taper mean, 1.19 [SE, .09]; P = .003) groups and from stabilization to post taper in the buprenorphine group (stabilization mean, 0.46 [SE, .05] post-taper mean, 1.17 [SE, .09]; P = .006), suggesting higher withdrawal for those groups during those periods. Naltrexone initiation was voluntary and the percentage of participants choosing naltrexone therapy within the clonidine (8 [22.2%]), tramadol ER (7 [19.4%]), or buprenorphine (3 [9.7%]) groups did not differ significantly (χ2 = 2.5, P = .29).
Conclusions and Relevance The results of this trial suggest that tramadol ER is more effective than clonidine and comparable to buprenorphine in reducing opioid withdrawal symptoms during a residential tapering program. Data support further examination of tramadol ER as a method to manage opioid withdrawal symptoms.
Trial Registration Clinicaltrials.gov Identifier: NCT01188421
中文翻译:

曲马多缓释片对阿片类药物戒断的疗效随机临床试验
重要的 阿片类药物使用障碍(OUD)是一个重大的公共卫生问题。使用可乐定或盐酸丁丙诺啡从阿片类药物监督撤药(即解毒)是一种广泛使用的治疗方法。
目的 评估盐酸曲马多缓释(ER)是一种批准的具有阿片类和非阿片类药物作用机制且滥用可能性低的止痛药,是否可有效用于有监督的戒断环境。
设计,环境和参与者 在103名OUD参与者的住宅研究环境中进行了一项随机临床试验。参与者的治疗通过每天4次皮下注射的30毫克吗啡来稳定。然后建立了使用可乐定(n = 36),曲马多ER(n = 36)或丁丙诺啡(n = 31)的7天锥度,并且在减量期后将患者转为双盲安慰剂。该研究于2010年10月25日至2015年6月23日进行。
主要结果和措施 保留,戒断症状管理,同时用药和纳曲酮诱导。随时间推移分析结果,并使用意向治疗组和完成者组的曲线下面积。
结果 在103名参与者中,男性88名(85.4%),白人43名(41.7%)。平均(SD)年龄为28.9(10.4)岁。与可乐定参与者(22 [61.1%])相比,丁丙诺啡参与者(28 [90.3%])在锥度末期被保留的可能性更高。曲马多ER滞留为中间,并没有不同于其它基团的显著差异(26 [72.2%];χ 2 = 8.5,P = 0.01)。撤离的时程分析显示,临床鸦片戒断量表(COWS)评分的阶段(锥度,锥度后)具有显着影响(锥度平均值为5.19 [SE,.26];锥度平均值为3.97 [SE,.23] ]; F 2,170 = 3.6,P = .03)和主观阿片类药物戒断量表(SOWS)得分(锥度平均值,8.81 [SE,.40];锥度平均值,4.14 [SE,.30];F 2,170 = 15.7,P <.001),但没有群效应或群×相相互作用。SOWS总分曲线下面积的分析显示 ,可乐定在锥度和锥度后期间的戒断严重程度显着降低(F 2,159 = 17.7,P <.001)(锥度平均值为13.1;锥度平均值为3.2;锥度平均值为3.2)。P <.001)和曲马多ER(锥度平均值为7.4;锥度平均值后为2.8; P = .03),但不是丁丙诺啡(锥度平均值为6.4;锥度平均值后为7.4)。伴随药物的使用显着增加(F 2,159 = 30.7, P <.001),可乐定从稳定到逐渐减小(稳定度平均值为0.64 [SE,.05];锥形平均值为1.54 [SE,.10];P <.001)和曲马多ER(稳定度平均值, 0.53 [SE,.05];锥度平均值,1.19 [SE,.09]; P = .003)组,丁丙诺啡组从稳定期到锥度后锥度(稳定度平均值,0.46 [SE,.05]锥度后平均值) ,1.17 [SE,.09];P = .006),表明这些时期内这些人群的退出率更高。纳曲酮开始是自愿的,参与者的可乐定中选择的纳曲酮治疗的百分比(8 [22.2%]),曲马多ER(7 [19.4%]),或叔丁啡(3 [9.7%])基团没有显著不同(χ 2 = 2.5,P = 0.29)。
结论与相关性 该试验的结果表明,在减少居民量的过程中,曲马多ER比可乐定更有效,与丁丙诺啡相当,可减轻阿片类药物的戒断症状。数据支持曲马多ER的进一步检查,作为治疗阿片类药物戒断症状的方法。
试用注册 Clinicaltrials.gov标识符:NCT01188421



























 京公网安备 11010802027423号
京公网安备 11010802027423号