European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.ejmech.2017.09.005 Anastasia V. Konysheva , Vladimir O. Nebogatikov , Irina A. Tolmacheva , Maxim V. Dmitriev , Victoria V. Grishko
|
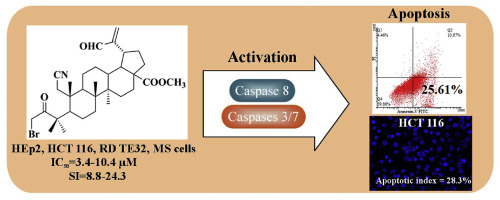
Extremely low content of biologically active triterpenoids with the fragmented or contracted ring A extractable from plants is the main disadvantage of their use in drug discovery and practical pharmacology. Development of new methods for synthesis of these compounds and their structural analogs from bioavailable triterpene precursors gives an opportunity to obtain promising agents for pharmacology with excellent yields. A new approach to synthesis of alkylated A-seco-triterpenoids, including the Beckmann fragmentation of 3-methyl-substituted allobetulin or betulinic acid methyl ester with 2-hydroxyimino group in the ring A was proposed. These compounds were used to prepare a series of 2,3-seco- and five-membered ring A lupane and oleanane derivatives, cytotoxicity of which was screened in vitro against the cancer (HEp-2, HCT 116, A549, RD TE32, MS) and non-cancerous (HEK 293) cell lines. Methyl 3-bromomethyl-1-cyano-3-oxo-2,3-seco-2-norlup-20(29)-en-30-al-28-oate was selected as the most active compound (IC50 3.4–10.4 μM for HEp-2, HCT 116, RD TE32, MS cells) capable of triggering caspase-8-mediated apoptosis in HCT 116 cells accompanied by typical apoptotic chromatin condensation, without any loss of mitochondrial membrane permeability.
中文翻译:

基于烷基化的2,3-seco-三萜类化合物的细胞毒性活性衍生物的合成
可从植物中提取的具有断裂或收缩的环A的生物活性三萜类化合物的含量极低,是其在药物发现和实用药理学中使用的主要缺点。从可生物利用的三萜烯前体合成这些化合物及其结构类似物的新方法的开发提供了以极好的收率获得有希望的药理学试剂的机会。提出了一种新的烷基化A-seco-三萜类化合物的合成方法,包括在环A中的3-甲基取代的别壁白蛋白或具有2-羟基亚氨基的桦木酸甲酯的贝克曼片段化。这些化合物用于制备一系列2,3-癸二元和五元环A的戊烷和齐墩果衍生物,其细胞毒性在体外进行了筛选抵抗癌细胞(HEp-2,HCT 116,A549,RD TE32,MS)和非癌细胞(HEK 293)。3-溴甲基-1-氰基-3-氧代-2,3-seco-2-norlup-20(29)-en-30-al-28-oate甲酯被选为最具活性的化合物(IC 50 3.4–10.4 μM为的HEp-2,HCT 116,RD TE32,MS细胞)能够触发胱天蛋白酶-8的-介导的凋亡在HCT 116个细胞伴随着典型的细胞凋亡染色质浓缩,而不线粒体膜通透性的任何损失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号