当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The formal synthesis of (+)-15-deoxy-Δ12,14-prostaglandin J2: Controlling exo-olefin geometry via SmI2-mediated cyclization
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.tetlet.2017.09.002 Kazunori Takahashi , Yuichi Arai , Toshio Honda
中文翻译:

的正式合成(+) - 15-脱氧Δ 12,14 -前列腺素Ĵ 2:管制外切烯烃几何经由SMI 2介导的环化
更新日期:2017-09-06
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.tetlet.2017.09.002 Kazunori Takahashi , Yuichi Arai , Toshio Honda
|
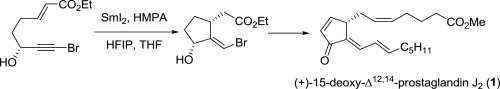
A synthetic approach of (+)-15d-PGJ2 has been developed. The present method features a stereoselective construction of the olefin unit using SmI2-mediated radical cyclization. The resulting cyclic compound was further utilized by efficient introduction of α and ω chains of prostaglandins to achieve enantioselective formal synthesis of (+)-15d-PGJ2.
中文翻译:

的正式合成(+) - 15-脱氧Δ 12,14 -前列腺素Ĵ 2:管制外切烯烃几何经由SMI 2介导的环化
已经开发出一种(+)-15d-PGJ 2的合成方法。本方法的特征在于使用SmI 2介导的自由基环化的烯烃单元的立体选择性构造。通过有效地引入前列腺素的α和ω链,进一步利用所得的环状化合物来实现(+)-15d-PGJ 2的对映选择性形式合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号