当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the novel inhibition mechanism of Apigenin to Pneumolysin by Molecular Modeling
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.cplett.2017.09.003 Xiaodi Niu , Yanan Yang , Meng Song , Guizhen Wang , Lin Sun , Yawen Gao , Hongsu Wang
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.cplett.2017.09.003 Xiaodi Niu , Yanan Yang , Meng Song , Guizhen Wang , Lin Sun , Yawen Gao , Hongsu Wang
|
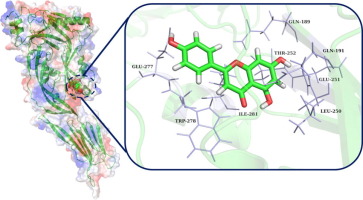
In this study, the mechanism of apigenin inhibition was explored using molecular modelling, binding energy calculation, and mutagenesis assays. Energy decomposition analysis indicated that apigenin binds in the gap between domains 3 and 4 of PLY. Using principal component analysis, we found that binding of apigenin to PLY weakens the motion of domains 3 and 4. Consequently, these domains cannot complete the transition from monomer to oligomer, thereby blocking oligomerisation of PLY and counteracting its haemolytic activity. This inhibitory mechanism was confirmed by haemolysis assays, and these findings will promote the future development of an antimicrobial agent.
中文翻译:

分子建模对芹菜素对肺炎球菌溶血素的新抑制机制的研究
在这项研究中,使用分子建模,结合能计算和诱变试验探索了芹菜素抑制的机理。能量分解分析表明芹菜素结合在PLY的结构域3和4之间的间隙中。使用主成分分析,我们发现芹菜素与PLY的结合会削弱结构域3和4的运动。因此,这些结构域无法完成从单体到低聚物的转变,从而阻止PLY的低聚并抵消其溶血活性。溶血分析证实了这种抑制机制,这些发现将促进抗菌剂的未来发展。
更新日期:2017-09-07
中文翻译:

分子建模对芹菜素对肺炎球菌溶血素的新抑制机制的研究
在这项研究中,使用分子建模,结合能计算和诱变试验探索了芹菜素抑制的机理。能量分解分析表明芹菜素结合在PLY的结构域3和4之间的间隙中。使用主成分分析,我们发现芹菜素与PLY的结合会削弱结构域3和4的运动。因此,这些结构域无法完成从单体到低聚物的转变,从而阻止PLY的低聚并抵消其溶血活性。溶血分析证实了这种抑制机制,这些发现将促进抗菌剂的未来发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号