当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical synthesis of ferrate(VI) using sponge iron anode and oxidative transformations of antibiotic and pesticide
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.jhazmat.2017.08.081 Xuhui Sun , Kexin Zu , He Liang , Lin Sun , Lingyun Zhang , Chuanyi Wang , Virender K. Sharma
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2017-09-06 , DOI: 10.1016/j.jhazmat.2017.08.081 Xuhui Sun , Kexin Zu , He Liang , Lin Sun , Lingyun Zhang , Chuanyi Wang , Virender K. Sharma
|
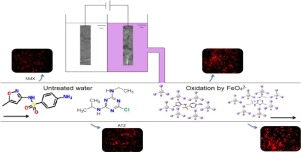
Passivation of anode is a main challenge in the electrochemical synthesis of ferrate(VI) (FeVIO42−, Fe(VI)). A series of eclectrochemical approaches were employed including polarization curve, cyclic voltammetry, and electrochemical impedance spectroscopy (EIS) to analyze the physicochemical processes involved in electrochemical synthesis of Fe(VI) using sponge iron and cast iron anodes. The results demonstrate that the sponge iron anode achieved higher yield of Fe(VI) compared to grey cast iron anode. The optimum condition to generate Fe(VI) using sponge iron was 35–50 °C and 30 mA/cm2. Significantly, the sponge iron anode could generate Fe(VI) for a long duration (>10 h) under these conditions; possibly suitable for large scale synthesis of Fe(VI). The prepared Fe(VI) solution was used to treat antibiotic (sulfamethoxazole (SMX)) and pesticide (atrazine (ATZ)) in water. At a molar ratio of Fe(VI) to SMX as 20:1 in the pH range from 5.0 to 9.0, almost complete oxidative transformation of SMX could be obtained. Comparatively, oxidative transformation of ATZ was incomplete (∼70%) even when [Fe(VI)]:[ATZ] = 87 at pH 5.0–9.0. Fluorescence spectra and cytotoxicity studies suggest that the oxidative transformation products of both SMX and ATZ possess lower toxicity than the parent antibiotic and pesticide, respectively.
中文翻译:

海绵铁阳极电化学合成高铁酸盐(VI)以及抗生素和农药的氧化转化
阳极的钝化是高铁酸盐(VI)(Fe VI O 4 2-,Fe(VI))电化学合成中的主要挑战。采用了一系列电化学方法,包括极化曲线,循环伏安法和电化学阻抗谱(EIS),以分析涉及使用海绵铁和铸铁阳极电化学合成Fe(VI)的物理化学过程。结果表明,与灰铸铁阳极相比,海绵铁阳极获得了更高的Fe(VI)收率。使用海绵铁生成Fe(VI)的最佳条件是35–50°C和30 mA / cm 2。值得注意的是,在这些条件下,海绵铁阳极可以长时间(> 10 h)生成Fe(VI);可能适用于大规模合成Fe(VI)。制备的Fe(VI)溶液用于在水中处理抗生素(磺胺甲恶唑(SMX))和农药(阿特拉津(ATZ))。在5.0至9.0的pH范围内,Fe(VI)与SMX的摩尔比为20:1时,几乎可以完成SMX的完全氧化转化。相比之下,即使在[Fe(VI)]:[ATZ] = pH 5.0-9.0时,ATZ的氧化转化也不完全(约70%)。荧光光谱和细胞毒性研究表明,SMX和ATZ的氧化转化产物的毒性分别低于母体抗生素和农药。
更新日期:2017-09-07
中文翻译:

海绵铁阳极电化学合成高铁酸盐(VI)以及抗生素和农药的氧化转化
阳极的钝化是高铁酸盐(VI)(Fe VI O 4 2-,Fe(VI))电化学合成中的主要挑战。采用了一系列电化学方法,包括极化曲线,循环伏安法和电化学阻抗谱(EIS),以分析涉及使用海绵铁和铸铁阳极电化学合成Fe(VI)的物理化学过程。结果表明,与灰铸铁阳极相比,海绵铁阳极获得了更高的Fe(VI)收率。使用海绵铁生成Fe(VI)的最佳条件是35–50°C和30 mA / cm 2。值得注意的是,在这些条件下,海绵铁阳极可以长时间(> 10 h)生成Fe(VI);可能适用于大规模合成Fe(VI)。制备的Fe(VI)溶液用于在水中处理抗生素(磺胺甲恶唑(SMX))和农药(阿特拉津(ATZ))。在5.0至9.0的pH范围内,Fe(VI)与SMX的摩尔比为20:1时,几乎可以完成SMX的完全氧化转化。相比之下,即使在[Fe(VI)]:[ATZ] = pH 5.0-9.0时,ATZ的氧化转化也不完全(约70%)。荧光光谱和细胞毒性研究表明,SMX和ATZ的氧化转化产物的毒性分别低于母体抗生素和农药。











































 京公网安备 11010802027423号
京公网安备 11010802027423号