当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Conjugate Addition Catalyzed by a Copper Phosphoramidite Complex: Computational and Experimental Exploration of Asymmetric Induction
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acscatal.7b01453 Ruchuta Ardkhean 1 , Philippe M. C. Roth 1 , Rebecca M. Maksymowicz 1 , Alex Curran 1 , Qian Peng 2 , Robert S. Paton 1 , Stephen P. Fletcher 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acscatal.7b01453 Ruchuta Ardkhean 1 , Philippe M. C. Roth 1 , Rebecca M. Maksymowicz 1 , Alex Curran 1 , Qian Peng 2 , Robert S. Paton 1 , Stephen P. Fletcher 1
Affiliation
|
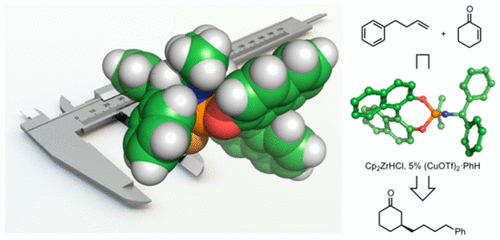
The stereochemical role of the phosphoramidite ligand in the asymmetric conjugate addition of alkylzirconium species to cyclic enones has been established through experimental and computational studies. Systematic, synthetic variation of the modular ligand established that the configuration of the binaphthol backbone is responsible for absolute stereocontrol, whereas modulation of the amido substituents leads to dramatic variations in the level of asymmetric induction. Chiral amido substituents are not required for enantioselectivity, leading to the discovery of a new family of easily synthesized phosphoramidites based on achiral amines that deliver equal levels of selectivity to Feringa’s ligand. A linear correlation between the length of the aromatic amido groups and experimentally determined enantioselectivity was uncovered for this class of ligand, which, following an optimization, led to highly selective ligands (up to 94% ee) with naphthyl rather than phenyl groups. An electronic effect of sterically similar aromatic substituents was investigated through NMR and DFT studies, showing that electron-rich aryl groups allow better Cu coordination. An interaction between the metal center and an aromatic group is responsible for this enhanced affinity and leads to a more tightly coordinated transition structure, leading to the major enantiomer. These studies illustrate the use of parametric quantitative structure–selectivity relationships to generate mechanistic models for asymmetric induction and catalyst structures that may be further probed by experiment and computation. This integrated approach leads to the rational modification of chiral ligands to achieve enhanced levels of selectivity.
中文翻译:

铜亚磷酰胺络合物催化的对映选择性共轭加成反应:不对称诱导的计算和实验探索
通过实验和计算研究,已经确定了亚磷酰胺配体在烷基锆物种向环烯酮的不对称共轭加成中的立体化学作用。模块化配体的系统,合成变化确定了联萘酚骨架的构型负责绝对立体控制,而酰胺基取代基的调节导致不对称诱导水平的显着变化。对映体选择性不需要手性酰胺基取代基,从而导致发现了一个新的容易合成的亚家族,其基于非手性胺,它们对Feringa的配体具有相同的选择性。对于这类配体,未发现芳族酰胺基团的长度与实验确定的对映选择性之间存在线性关系,经过优化后,可得到具有萘基而不是苯基的高选择性配体(至多94%ee)。通过NMR和DFT研究,研究了空间相似的芳族取代基的电子效应,结果表明,富含电子的芳基可实现更好的Cu配位。金属中心和芳族基团之间的相互作用是这种增强的亲和力的原因,并导致更紧密配位的过渡结构,从而形成主要的对映异构体。这些研究说明了使用参数化定量结构-选择性关系生成不对称感应和催化剂结构的机理模型,可以通过实验和计算来进一步探究。这种集成的方法导致手性配体的合理修饰,以实现更高水平的选择性。
更新日期:2017-09-07
中文翻译:

铜亚磷酰胺络合物催化的对映选择性共轭加成反应:不对称诱导的计算和实验探索
通过实验和计算研究,已经确定了亚磷酰胺配体在烷基锆物种向环烯酮的不对称共轭加成中的立体化学作用。模块化配体的系统,合成变化确定了联萘酚骨架的构型负责绝对立体控制,而酰胺基取代基的调节导致不对称诱导水平的显着变化。对映体选择性不需要手性酰胺基取代基,从而导致发现了一个新的容易合成的亚家族,其基于非手性胺,它们对Feringa的配体具有相同的选择性。对于这类配体,未发现芳族酰胺基团的长度与实验确定的对映选择性之间存在线性关系,经过优化后,可得到具有萘基而不是苯基的高选择性配体(至多94%ee)。通过NMR和DFT研究,研究了空间相似的芳族取代基的电子效应,结果表明,富含电子的芳基可实现更好的Cu配位。金属中心和芳族基团之间的相互作用是这种增强的亲和力的原因,并导致更紧密配位的过渡结构,从而形成主要的对映异构体。这些研究说明了使用参数化定量结构-选择性关系生成不对称感应和催化剂结构的机理模型,可以通过实验和计算来进一步探究。这种集成的方法导致手性配体的合理修饰,以实现更高水平的选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号