当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Targeting by a Mechanism-Based Inactivator against Pyridoxal 5′-Phosphate-Dependent Enzymes: Mechanisms of Inactivation and Alternative Turnover
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.biochem.7b00499 Romila Mascarenhas 1 , Hoang V. Le 2, 3 , Kenneth D. Clevenger 2 , Helaina J. Lehrer 4 , Dagmar Ringe 4 , Neil L. Kelleher 2 , Richard B. Silverman 2 , Dali Liu 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.biochem.7b00499 Romila Mascarenhas 1 , Hoang V. Le 2, 3 , Kenneth D. Clevenger 2 , Helaina J. Lehrer 4 , Dagmar Ringe 4 , Neil L. Kelleher 2 , Richard B. Silverman 2 , Dali Liu 1
Affiliation
|
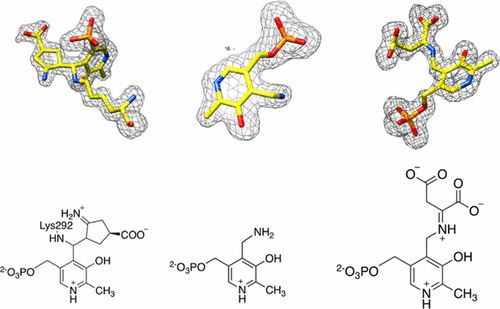
Potent mechanism-based inactivators can be rationally designed against pyridoxal 5′-phosphate (PLP)-dependent drug targets, such as ornithine aminotransferase (OAT) or γ-aminobutyric acid aminotransferase (GABA-AT). An important challenge, however, is the lack of selectivity toward other PLP-dependent, off-target enzymes, because of similarities in mechanisms of all PLP-dependent aminotransferase reactions. On the basis of complex crystal structures, we investigate the inactivation mechanism of OAT, a hepatocellular carcinoma target, by (1R,3S,4S)-3-amino-4-fluorocyclopentane-1-carboxylic acid (FCP), a known inactivator of GABA-AT. A crystal structure of OAT and FCP showed the formation of a ternary adduct. This adduct can be rationalized as occurring via an enamine mechanism of inactivation, similar to that reported for GABA-AT. However, the crystal structure of an off-target, PLP-dependent enzyme, aspartate aminotransferase (Asp-AT), in complex with FCP, along with the results of attempted inhibition assays, suggests that FCP is not an inactivator of Asp-AT, but rather an alternate substrate. Turnover of FCP by Asp-AT is also supported by high-resolution mass spectrometry. Amid existing difficulties in achieving selectivity of inactivation among a large number of PLP-dependent enzymes, the obtained results provide evidence that a desirable selectivity could be achieved, taking advantage of subtle structural and mechanistic differences between a drug-target enzyme and an off-target enzyme, despite their largely similar substrate binding sites and catalytic mechanisms.
中文翻译:

基于机制的灭活剂对吡咯醛5'-磷酸盐依赖性酶的选择性靶向:灭活和替代周转的机制
可以合理设计基于有效机理的灭活剂,以抗依赖吡ido醛5'-磷酸(PLP)的药物靶标,例如鸟氨酸氨基转移酶(OAT)或γ-氨基丁酸氨基转移酶(GABA-AT)。然而,一个重要的挑战是,由于所有PLP依赖性氨基转移酶反应机理相似,因此对其他PLP依赖性脱靶酶缺乏选择性。在复杂的晶体结构的基础上,我们通过(1 R,3 S,4 S)-3-氨基-4-氟环戊烷-1-羧酸(FCP),一种已知的GABA-AT灭活剂。OAT和FCP的晶体结构表明形成了三元加合物。该加合物可以合理地认为是通过烯胺失活机理发生的,类似于针对GABA-AT的报道。但是,脱靶,依赖PLP的酶,天冬氨酸转氨酶(Asp-AT)的晶体结构与FCP结合,以及尝试的抑制试验结果表明,FCP不是Asp-AT的灭活剂,而是另一种基材。高分辨率质谱也支持Asp-AT进行FCP的周转。在实现众多PLP依赖性酶之间灭活的选择性方面存在困难的情况下,获得的结果提供了证据,表明可以实现理想的选择性,
更新日期:2017-09-06
中文翻译:

基于机制的灭活剂对吡咯醛5'-磷酸盐依赖性酶的选择性靶向:灭活和替代周转的机制
可以合理设计基于有效机理的灭活剂,以抗依赖吡ido醛5'-磷酸(PLP)的药物靶标,例如鸟氨酸氨基转移酶(OAT)或γ-氨基丁酸氨基转移酶(GABA-AT)。然而,一个重要的挑战是,由于所有PLP依赖性氨基转移酶反应机理相似,因此对其他PLP依赖性脱靶酶缺乏选择性。在复杂的晶体结构的基础上,我们通过(1 R,3 S,4 S)-3-氨基-4-氟环戊烷-1-羧酸(FCP),一种已知的GABA-AT灭活剂。OAT和FCP的晶体结构表明形成了三元加合物。该加合物可以合理地认为是通过烯胺失活机理发生的,类似于针对GABA-AT的报道。但是,脱靶,依赖PLP的酶,天冬氨酸转氨酶(Asp-AT)的晶体结构与FCP结合,以及尝试的抑制试验结果表明,FCP不是Asp-AT的灭活剂,而是另一种基材。高分辨率质谱也支持Asp-AT进行FCP的周转。在实现众多PLP依赖性酶之间灭活的选择性方面存在困难的情况下,获得的结果提供了证据,表明可以实现理想的选择性,



























 京公网安备 11010802027423号
京公网安备 11010802027423号