当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of Sporadic Parkinson Disease Associated Mutations A18T and A29S in Enhanced α-Synuclein Fibrillation and Cytotoxicity
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acschemneuro.6b00430 Sanjay Kumar 1, 2 , Deepak Kumar Jangir 1 , Roshan Kumar 1, 2 , Manisha Kumari 1 , Neel Sarovar Bhavesh 3 , Tushar Kanti Maiti 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acschemneuro.6b00430 Sanjay Kumar 1, 2 , Deepak Kumar Jangir 1 , Roshan Kumar 1, 2 , Manisha Kumari 1 , Neel Sarovar Bhavesh 3 , Tushar Kanti Maiti 1
Affiliation
|
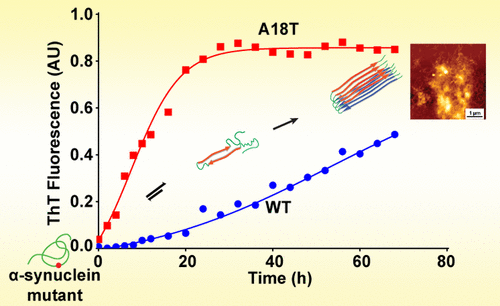
Deposition of presynaptic protein α-synuclein in Lewy bodies and Lewy neurites in the substantia nigra region of brain has been linked with the clinical symptoms of the Parkinson’s disease (PD). Proteotoxic stress conditions and mutations that cause abnormal aggregation of α-synuclein have close association with onset of PD and its progression. Therefore, studies pertaining to α-synuclein mutations play important roles in mechanistic understanding of aggregation behavior of the protein and subsequent pathology. Herein, guided by this fact, we have studied the aggregation kinetics, morphology, and neurotoxic effects of the two newly discovered sporadic PD associated mutants A18T and A29S of α-synuclein. Our studies demonstrate that both of the mutants are aggregation prone and undergo rapid aggregation compared to wild-type α-synuclein. Further, it was found that A18T mutant followed faster aggregation kinetics compared to A29S substitution. Additionally, we have designed three point mutations of α-synuclein for better understanding of the effects of substitutions on protein aggregation and demonstrated that substitution of alanine at the 18th position is highly sensitive compared to adjacent positions. Our results provide better understanding of the effects of α-synuclein mutations on its aggregation behavior that may be important in development of PD pathology.
中文翻译:

散发性帕金森病相关突变A18T和A29S在增强的α-突触核蛋白纤颤和细胞毒性中的作用
路易体和脑黑质区路易神经突中突触前蛋白α-突触核蛋白的沉积与帕金森氏病(PD)的临床症状有关。蛋白毒性应激条件和引起α-突触核蛋白异常聚集的突变与PD的发作及其进展密切相关。因此,有关α-突触核蛋白突变的研究在蛋白质的聚集行为和随后的病理学的机理理解中起着重要的作用。在此,根据这一事实,我们研究了两个新发现的散发的PD相关的α-突触核蛋白的突变体A18T和A29S的聚集动力学,形态和神经毒性作用。我们的研究表明,与野生型α-突触核蛋白相比,这两个突变体都易于聚集,并且经历了快速聚集。进一步,发现与A29S取代相比,A18T突变体遵循更快的聚集动力学。此外,我们设计了α-突触核蛋白的三个点突变,以更好地了解取代对蛋白质聚集的影响,并证明了与相邻位置相比,第18位丙氨酸的取代高度敏感。我们的结果提供了更好的了解α-突触核蛋白突变对其聚集行为的影响,这可能对PD病理学的发展很重要。我们设计了α-突触核蛋白的三个点突变,以更好地了解取代对蛋白质聚集的影响,并证明了与相邻位置相比,第18位丙氨酸的取代高度敏感。我们的结果提供了更好的了解α-突触核蛋白突变对其聚集行为的影响,这可能对PD病理学的发展很重要。我们设计了α-突触核蛋白的三个点突变,以更好地了解取代对蛋白质聚集的影响,并证明了与相邻位置相比,第18位丙氨酸的取代高度敏感。我们的结果提供了更好的了解α-突触核蛋白突变对其聚集行为的影响,这可能对PD病理学的发展很重要。
更新日期:2017-09-06
中文翻译:

散发性帕金森病相关突变A18T和A29S在增强的α-突触核蛋白纤颤和细胞毒性中的作用
路易体和脑黑质区路易神经突中突触前蛋白α-突触核蛋白的沉积与帕金森氏病(PD)的临床症状有关。蛋白毒性应激条件和引起α-突触核蛋白异常聚集的突变与PD的发作及其进展密切相关。因此,有关α-突触核蛋白突变的研究在蛋白质的聚集行为和随后的病理学的机理理解中起着重要的作用。在此,根据这一事实,我们研究了两个新发现的散发的PD相关的α-突触核蛋白的突变体A18T和A29S的聚集动力学,形态和神经毒性作用。我们的研究表明,与野生型α-突触核蛋白相比,这两个突变体都易于聚集,并且经历了快速聚集。进一步,发现与A29S取代相比,A18T突变体遵循更快的聚集动力学。此外,我们设计了α-突触核蛋白的三个点突变,以更好地了解取代对蛋白质聚集的影响,并证明了与相邻位置相比,第18位丙氨酸的取代高度敏感。我们的结果提供了更好的了解α-突触核蛋白突变对其聚集行为的影响,这可能对PD病理学的发展很重要。我们设计了α-突触核蛋白的三个点突变,以更好地了解取代对蛋白质聚集的影响,并证明了与相邻位置相比,第18位丙氨酸的取代高度敏感。我们的结果提供了更好的了解α-突触核蛋白突变对其聚集行为的影响,这可能对PD病理学的发展很重要。我们设计了α-突触核蛋白的三个点突变,以更好地了解取代对蛋白质聚集的影响,并证明了与相邻位置相比,第18位丙氨酸的取代高度敏感。我们的结果提供了更好的了解α-突触核蛋白突变对其聚集行为的影响,这可能对PD病理学的发展很重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号