当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the role of the alkali cations on methanol thiolation
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7cy01255a Ricardo Bermejo-Deval 1, 2, 3, 4 , Raimund M. H. Walter 1, 2, 3, 4 , Oliver Y. Gutiérrez 1, 2, 3, 4 , Johannes A. Lercher 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7cy01255a Ricardo Bermejo-Deval 1, 2, 3, 4 , Raimund M. H. Walter 1, 2, 3, 4 , Oliver Y. Gutiérrez 1, 2, 3, 4 , Johannes A. Lercher 1, 2, 3, 4, 5
Affiliation
|
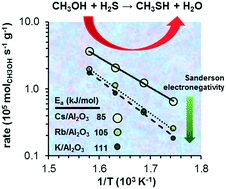
The electronegativity effect of the alkali cations on the formation of methanethiol by reaction of methanol and H2S was studied with K+, Rb+, and Cs+ supported on γ-Al2O3. Catalyst activation in H2S led to the formation of sulfur oxyanions, including SO3−2, S2O3−2, SO4−2 and S2O6−2. The surface modified aluminas have strong basic properties. Accessible alkali cations on γ-Al2O3 are the active sites catalyzing the SN2 nucleophilic substitution. These sites stabilize two Lewis acid–base pairs, formed upon H2S and methanol dissociation, in proximity. Decreasing electronegativity of the alkali enhances the concentration of basic sites and their adsorption capacity. In consequence, the rates of methanethiol formation increased in the sequence K/γ-Al2O3 < Rb/γ-Al2O3 < Cs/γ-Al2O3. The activation energies also decreased in parallel with the decreasing electronegativity of the alkali, which is attributed to the increasing electron density at the SH− groups that facilitates the nucleophilic attack.
中文翻译:

碱性阳离子对甲醇硫醇化的作用
以γ-Al 2 O 3为载体的K +,Rb +和Cs +,研究了碱金属阳离子对甲醇与H 2 S反应生成甲硫醇的电负性的影响。H 2 S中的催化剂活化导致形成硫氧阴离子,包括SO 3 -2,S 2 O 3 -2,SO 4 -2和S 2 O 6 -2。表面改性的氧化铝具有很强的基本性能。上无障碍的碱金属阳离子的γ-Al 2 ö 3是催化SN 2亲核取代的活性位点。这些位点稳定了在H 2 S和甲醇离解时形成的两个路易斯酸碱对。碱的电负性降低会增加碱性位点的浓度及其吸附能力。结果,甲硫醇的形成率的序列中增加K /γ-Al系2 ö 3 <RB /γ-Al系2 ö 3 <CS /γ-Al系2 ö 3。活化能也减少平行于碱,这是在SH归因于增加电子密度的降低电- 促进亲核攻击的基团。
更新日期:2017-09-06
中文翻译:

碱性阳离子对甲醇硫醇化的作用
以γ-Al 2 O 3为载体的K +,Rb +和Cs +,研究了碱金属阳离子对甲醇与H 2 S反应生成甲硫醇的电负性的影响。H 2 S中的催化剂活化导致形成硫氧阴离子,包括SO 3 -2,S 2 O 3 -2,SO 4 -2和S 2 O 6 -2。表面改性的氧化铝具有很强的基本性能。上无障碍的碱金属阳离子的γ-Al 2 ö 3是催化SN 2亲核取代的活性位点。这些位点稳定了在H 2 S和甲醇离解时形成的两个路易斯酸碱对。碱的电负性降低会增加碱性位点的浓度及其吸附能力。结果,甲硫醇的形成率的序列中增加K /γ-Al系2 ö 3 <RB /γ-Al系2 ö 3 <CS /γ-Al系2 ö 3。活化能也减少平行于碱,这是在SH归因于增加电子密度的降低电- 促进亲核攻击的基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号