当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific Conjugation to Native and Engineered Lysines in Human Immunoglobulins by Microbial Transglutaminase
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00439 Jared L. Spidel 1 , Benjamin Vaessen 1 , Earl F. Albone 1 , Xin Cheng 1 , Arielle Verdi 1 , J. Bradford Kline 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00439 Jared L. Spidel 1 , Benjamin Vaessen 1 , Earl F. Albone 1 , Xin Cheng 1 , Arielle Verdi 1 , J. Bradford Kline 1
Affiliation
|
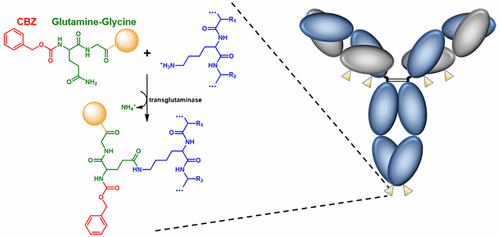
The use of microbial transglutaminase (MTG) to produce site-specific antibody–drug conjugates (ADCs) has thus far focused on the transamidation of engineered acyl donor glutamine residues in an antibody based on the hypothesis that the lower specificity of MTG for acyl acceptor lysines may result in the transamidation of multiple native lysine residues, thereby yielding heterogeneous products. We investigated the utilization of native IgG lysines as acyl acceptor sites for glutamine-based acyl donor substrates. Of the approximately 80 lysines in multiple recombinant IgG monoclonal antibodies (mAbs), none were transamidated. Because recombinant mAbs lack the C-terminal Lys447 due to cleavage by carboxypeptidase B in the production cell host, we explored whether blocking the cleavage of Lys447 by the addition of a C-terminal amino acid could result in transamidation of Lys447 by a variety of acyl donor substrates. MTG efficiently transamidated Lys447 in the presence of any nonacidic, nonproline amino acid residue at position 448. Lysine scanning mutagenesis throughout the antibody further revealed several transamidation sites in both the heavy- and light-chain constant regions. Additionally, scanning mutagenesis of the hinge region in a Fab′ fragment revealed sites of transamidation that were not reactive in the context of the full-length mAb. Here, we demonstrate the utility of single lysine substitutions and the C-terminal Lys447 for engineering efficient acyl acceptor sites suitable for site-specific conjugation to a range of glutamine-based acyl donor substrates.
中文翻译:

微生物转谷氨酰胺酶与人免疫球蛋白中天然和工程化赖氨酸的位点特异性缀合
迄今为止,利用微生物转谷氨酰胺酶(MTG)产生位点特异性抗体-药物偶联物(ADC)的研究重点在于抗体中工程改造的酰基供体谷氨酰胺残基的转酰胺基化,其假设是MTG对酰基受体赖氨酸的特异性较低可能导致多个天然赖氨酸残基的转酰胺基化,从而产生异质产物。我们调查了天然IgG赖氨酸作为基于谷氨酰胺的酰基供体底物的酰基受体位点的利用。在多种重组IgG单克隆抗体(mAb)中大约80个赖氨酸中,没有一个被转酰胺基化。由于重组单克隆抗体由于在生产细胞宿主中被羧肽酶B裂解而缺乏C端Lys447,我们探讨了通过添加C末端氨基酸来阻断Lys447的裂解是否会导致各种酰基供体底物对Lys447的转酰胺基作用。在位置448处存在任何非酸性,非脯氨酸氨基酸残基的情况下,MTG有效地转酰胺化了Lys447。整个抗体中的赖氨酸扫描诱变进一步揭示了重链和轻链恒定区中的多个转酰胺基位。另外,对Fab'片段中的铰链区的诱变的扫描揭示了在全长mAb的情况下不发生反应的转酰胺基化位点。在这里,我们证明了单个赖氨酸取代和C末端Lys447的实用性,可用于工程改造有效的酰基受体位点,适用于特定位点缀合到一系列基于谷氨酰胺的酰基供体底物上。
更新日期:2017-09-06
中文翻译:

微生物转谷氨酰胺酶与人免疫球蛋白中天然和工程化赖氨酸的位点特异性缀合
迄今为止,利用微生物转谷氨酰胺酶(MTG)产生位点特异性抗体-药物偶联物(ADC)的研究重点在于抗体中工程改造的酰基供体谷氨酰胺残基的转酰胺基化,其假设是MTG对酰基受体赖氨酸的特异性较低可能导致多个天然赖氨酸残基的转酰胺基化,从而产生异质产物。我们调查了天然IgG赖氨酸作为基于谷氨酰胺的酰基供体底物的酰基受体位点的利用。在多种重组IgG单克隆抗体(mAb)中大约80个赖氨酸中,没有一个被转酰胺基化。由于重组单克隆抗体由于在生产细胞宿主中被羧肽酶B裂解而缺乏C端Lys447,我们探讨了通过添加C末端氨基酸来阻断Lys447的裂解是否会导致各种酰基供体底物对Lys447的转酰胺基作用。在位置448处存在任何非酸性,非脯氨酸氨基酸残基的情况下,MTG有效地转酰胺化了Lys447。整个抗体中的赖氨酸扫描诱变进一步揭示了重链和轻链恒定区中的多个转酰胺基位。另外,对Fab'片段中的铰链区的诱变的扫描揭示了在全长mAb的情况下不发生反应的转酰胺基化位点。在这里,我们证明了单个赖氨酸取代和C末端Lys447的实用性,可用于工程改造有效的酰基受体位点,适用于特定位点缀合到一系列基于谷氨酰胺的酰基供体底物上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号