当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Salt, Pressure, and Water Activity on Homogeneous Ice Nucleation
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.jpclett.7b01551 Jorge R. Espinosa 1 , Guiomar D. Soria 1 , Jorge Ramirez 2 , Chantal Valeriani 3 , Carlos Vega 1 , Eduardo Sanz 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.jpclett.7b01551 Jorge R. Espinosa 1 , Guiomar D. Soria 1 , Jorge Ramirez 2 , Chantal Valeriani 3 , Carlos Vega 1 , Eduardo Sanz 1
Affiliation
|
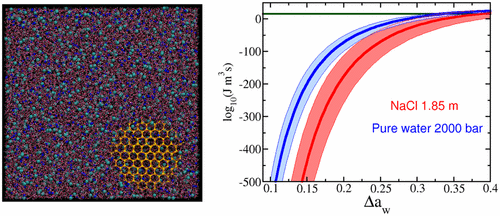
Pure water can be substantially supercooled below the melting temperature without transforming into ice. The achievable supercooling can be enhanced by adding solutes or by applying hydrostatic pressure. Avoiding ice formation is of great importance in the cryopreservation of food or biological samples. In this Letter, we investigate the similarity between the effects of pressure and salt on ice formation using a combination of state-of-the-art simulation techniques. We find that both hinder ice formation by increasing the energetic cost of creating the ice–fluid interface. Moreover, we examine the widely accepted proposal that the ice nucleation rate for different pressures and solute concentrations can be mapped through the activity of water [Koop, L.; Tsias, P. Nature, 2000, 406, 611]. We show that such a proposal is not consistent with the nucleation rates predicted in our simulations because it does not include all parameters affecting ice nucleation. Therefore, even though salt and pressure have a qualitatively similar effect on ice formation, they cannot be quantitatively mapped onto one another.
中文翻译:

盐,压力和水活度在均质冰核中的作用
纯水可在低于融化温度的条件下充分过冷而不会转变为冰。可以通过添加溶质或施加静水压力来提高可达到的过冷度。在食品或生物样品的冷冻保存中,避免结冰非常重要。在这封信中,我们将结合最先进的模拟技术,研究压力和盐对冰形成的影响之间的相似性。我们发现两者都通过增加创建冰-流体界面的能量成本而阻碍了冰的形成。此外,我们研究了被广泛接受的建议,即可以通过水的活度绘制不同压力和溶质浓度下的冰成核率[库普湖 Tsias,P。 自然,2000,406,611]。我们表明,这样的建议与我们的模拟中预测的成核速率不一致,因为它没有包括影响冰成核的所有参数。因此,即使盐和压力对结冰的影响在质量上相似,也无法将它们定量地相互映射。
更新日期:2017-09-06
中文翻译:

盐,压力和水活度在均质冰核中的作用
纯水可在低于融化温度的条件下充分过冷而不会转变为冰。可以通过添加溶质或施加静水压力来提高可达到的过冷度。在食品或生物样品的冷冻保存中,避免结冰非常重要。在这封信中,我们将结合最先进的模拟技术,研究压力和盐对冰形成的影响之间的相似性。我们发现两者都通过增加创建冰-流体界面的能量成本而阻碍了冰的形成。此外,我们研究了被广泛接受的建议,即可以通过水的活度绘制不同压力和溶质浓度下的冰成核率[











































 京公网安备 11010802027423号
京公网安备 11010802027423号