当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation
Journal of Hepatology ( IF 26.8 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.jhep.2017.06.015 Mark Kin Fai Ma , Eunice Yuen Ting Lau , Doris Hoi Wing Leung , Jessica Lo , Nicole Pui Yu Ho , Lily Kwan Wai Cheng , Stephanie Ma , Chi Ho Lin , John A. Copland , Jin Ding , Regina Cheuk Lam Lo , Irene Oi Lin Ng , Terence Kin Wah Lee
Journal of Hepatology ( IF 26.8 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.jhep.2017.06.015 Mark Kin Fai Ma , Eunice Yuen Ting Lau , Doris Hoi Wing Leung , Jessica Lo , Nicole Pui Yu Ho , Lily Kwan Wai Cheng , Stephanie Ma , Chi Ho Lin , John A. Copland , Jin Ding , Regina Cheuk Lam Lo , Irene Oi Lin Ng , Terence Kin Wah Lee
|
BACKGROUND & AIMS
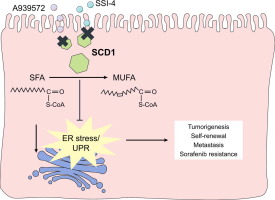
We investigated the functional role and clinical significance of stearoyl-CoA desaturase-1 (SCD1) mediated endoplasmic reticulum (ER) stress in regulating liver tumor-initiating cells (T-ICs) and sorafenib resistance, with the aim of developing a novel therapeutic strategy against hepatocellular carcinomas (HCCs). METHODS
We evaluated the clinic-pathological relevance of SCD1 and its correlation with sorafenib resistance in large cohorts of HCC clinical samples by qPCR and immunohistochemical analyses. Lentiviral-based overexpression and knockdown approaches were performed to characterize the functional roles of SCD1 in regulating liver T-ICs and sorafenib resistance. Molecular pathways mediating the phenotypic alterations were identified through RNA sequencing analysis and functional rescue experiments. The combinatorial effect of SCD1 inhibition and sorafenib was tested using a patient-derived tumor xenograft (PDTX) model. RESULTS
SCD1 overexpression was found in HCC, which was associated with shorter disease-free survival (p = 0.008, log rank test). SCD1 was found to regulate the populations of liver T-ICs; while its suppression by a SCD1 inhibitor suppressed liver T-ICs and sorafenib resistance. Interestingly, SCD1 was markedly upregulated in our established sorafenib-resistant PDTX model, and its overexpression predicts the clinical response of HCC patients to sorafenib treatment. Suppression of SCD1 forces liver T-ICs to differentiate via ER stress-induced unfolded protein response, resulting in an enhanced sensitivity to sorafenib. The PDTX#1 model, combined with sorafenib treatment and a novel SCD1 inhibitor (SSI-4), showed a maximal growth suppressive effect. CONCLUSIONS
SCD1-mediated ER stress regulates liver T-ICs and sorafenib sensitivity. Targeting SCD1 alone or in combination with sorafenib might be a novel personalized medicine against HCC. Lay summary: In this study, SCD1 was found to play a critical role in regulating liver tumor-initiating cells and sorafenib resistance through the regulation of ER stress-mediated differentiation. Targeting SCD1 in combination with sorafenib may be a novel therapeutic strategy against liver cancer.
中文翻译:

硬脂酰辅酶A去饱和酶通过调节内质网应激诱导的分化来调节索拉非尼抵抗
背景和目的 我们研究了硬脂酰辅酶 A 去饱和酶-1 (SCD1) 介导的内质网 (ER) 应激在调节肝肿瘤起始细胞 (T-IC) 和索拉非尼耐药性中的功能作用和临床意义,目的是开发一种针对肝细胞癌 (HCC) 的新治疗策略。方法 我们通过 qPCR 和免疫组织化学分析在大量 HCC 临床样本中评估了 SCD1 的临床病理相关性及其与索拉非尼耐药性的相关性。进行了基于慢病毒的过表达和敲低方法,以表征 SCD1 在调节肝脏 T-IC 和索拉非尼耐药中的功能作用。通过 RNA 测序分析和功能拯救实验确定了介导表型改变的分子途径。使用源自患者的肿瘤异种移植物 (PDTX) 模型测试了 SCD1 抑制和索拉非尼的组合效应。结果 在 HCC 中发现 SCD1 过表达,这与较短的无病生存期相关(p = 0.008,对数秩检验)。发现 SCD1 可调节肝脏 T-IC 的数量;而其被 SCD1 抑制剂的抑制抑制了肝脏 T-IC 和索拉非尼耐药性。有趣的是,SCD1 在我们建立的索拉非尼耐药 PDTX 模型中显着上调,其过表达预测了 HCC 患者对索拉非尼治疗的临床反应。SCD1 的抑制迫使肝脏 T-IC 通过 ER 应激诱导的未折叠蛋白反应进行分化,导致对索拉非尼的敏感性增强。PDTX#1 模型,结合索拉非尼治疗和新型 SCD1 抑制剂(SSI-4),表现出最大的生长抑制作用。结论 SCD1 介导的内质网应激调节肝脏 T-IC 和索拉非尼敏感性。单独或与索拉非尼联合靶向 SCD1 可能是一种针对 HCC 的新型个性化药物。小结:在本研究中,发现 SCD1 通过调节内质网应激介导的分化,在调节肝脏肿瘤起始细胞和索拉非尼耐药中发挥关键作用。靶向 SCD1 联合索拉非尼可能是一种新的肝癌治疗策略。发现 SCD1 通过调节内质网应激介导的分化,在调节肝脏肿瘤起始细胞和索拉非尼耐药性方面发挥关键作用。靶向 SCD1 联合索拉非尼可能是一种新的肝癌治疗策略。发现 SCD1 通过调节内质网应激介导的分化,在调节肝脏肿瘤起始细胞和索拉非尼耐药性方面发挥关键作用。靶向 SCD1 联合索拉非尼可能是一种新的肝癌治疗策略。
更新日期:2017-11-01
中文翻译:

硬脂酰辅酶A去饱和酶通过调节内质网应激诱导的分化来调节索拉非尼抵抗
背景和目的 我们研究了硬脂酰辅酶 A 去饱和酶-1 (SCD1) 介导的内质网 (ER) 应激在调节肝肿瘤起始细胞 (T-IC) 和索拉非尼耐药性中的功能作用和临床意义,目的是开发一种针对肝细胞癌 (HCC) 的新治疗策略。方法 我们通过 qPCR 和免疫组织化学分析在大量 HCC 临床样本中评估了 SCD1 的临床病理相关性及其与索拉非尼耐药性的相关性。进行了基于慢病毒的过表达和敲低方法,以表征 SCD1 在调节肝脏 T-IC 和索拉非尼耐药中的功能作用。通过 RNA 测序分析和功能拯救实验确定了介导表型改变的分子途径。使用源自患者的肿瘤异种移植物 (PDTX) 模型测试了 SCD1 抑制和索拉非尼的组合效应。结果 在 HCC 中发现 SCD1 过表达,这与较短的无病生存期相关(p = 0.008,对数秩检验)。发现 SCD1 可调节肝脏 T-IC 的数量;而其被 SCD1 抑制剂的抑制抑制了肝脏 T-IC 和索拉非尼耐药性。有趣的是,SCD1 在我们建立的索拉非尼耐药 PDTX 模型中显着上调,其过表达预测了 HCC 患者对索拉非尼治疗的临床反应。SCD1 的抑制迫使肝脏 T-IC 通过 ER 应激诱导的未折叠蛋白反应进行分化,导致对索拉非尼的敏感性增强。PDTX#1 模型,结合索拉非尼治疗和新型 SCD1 抑制剂(SSI-4),表现出最大的生长抑制作用。结论 SCD1 介导的内质网应激调节肝脏 T-IC 和索拉非尼敏感性。单独或与索拉非尼联合靶向 SCD1 可能是一种针对 HCC 的新型个性化药物。小结:在本研究中,发现 SCD1 通过调节内质网应激介导的分化,在调节肝脏肿瘤起始细胞和索拉非尼耐药中发挥关键作用。靶向 SCD1 联合索拉非尼可能是一种新的肝癌治疗策略。发现 SCD1 通过调节内质网应激介导的分化,在调节肝脏肿瘤起始细胞和索拉非尼耐药性方面发挥关键作用。靶向 SCD1 联合索拉非尼可能是一种新的肝癌治疗策略。发现 SCD1 通过调节内质网应激介导的分化,在调节肝脏肿瘤起始细胞和索拉非尼耐药性方面发挥关键作用。靶向 SCD1 联合索拉非尼可能是一种新的肝癌治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号