当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety of the 2D/3D direct-acting antiviral regimen in HCV-induced Child-Pugh A cirrhosis – A pooled analysis
Journal of Hepatology ( IF 26.8 ) Pub Date : 2017-10-01 , DOI: 10.1016/j.jhep.2017.06.011 Fred Poordad 1 , David R Nelson 2 , Jordan J Feld 3 , Michael W Fried 4 , Heiner Wedemeyer 5 , Lois Larsen 6 , Daniel E Cohen 6 , Eric Cohen 6 , Niloufar Mobashery 6 , Fernando Tatsch 6 , Graham R Foster 7
Journal of Hepatology ( IF 26.8 ) Pub Date : 2017-10-01 , DOI: 10.1016/j.jhep.2017.06.011 Fred Poordad 1 , David R Nelson 2 , Jordan J Feld 3 , Michael W Fried 4 , Heiner Wedemeyer 5 , Lois Larsen 6 , Daniel E Cohen 6 , Eric Cohen 6 , Niloufar Mobashery 6 , Fernando Tatsch 6 , Graham R Foster 7
Affiliation
|
BACKGROUND & AIMS
Chronic hepatitis C virus (HCV)-infected patients with cirrhosis are a high-priority population for treatment. To help inform the benefit-risk profile of the all-oral direct-acting antiviral (DAA) combination regimen of ombitasvir, paritaprevir, and ritonavir, with or without dasabuvir (OBV/PTV/r±DSV) in patients with Child-Pugh A cirrhosis, we undertook a comprehensive review of AbbVie-sponsored clinical trials enrolling patients with Child-Pugh A cirrhosis. METHODS
Twelve phase II or III clinical trials of the 2-DAA regimen of OBV/PTV/r±ribavirin (RBV) or the 3-DAA regimen of OBV/PTV/r+DSV±RBV that included patients with Child-Pugh A cirrhosis were reviewed; patients who completed treatment by November 16, 2015 were included in a pooled, post hoc safety assessment. The number and percentage of patients with treatment-emergent adverse events (TEAEs), serious TEAEs, and TEAEs consistent with hepatic decompensation were reported. RESULTS
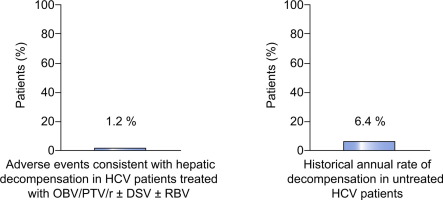
In 1,066 patients with Child-Pugh A cirrhosis, rates of serious TEAEs and TEAEs leading to study drug discontinuation were 5.3% (95% confidence interval [CI]: 4.1-6.8) and 2.2% (95% CI: 1.4-3.2), respectively. Thirteen patients (1.2%; 95% CI: 0.7-2.1) had a TEAE that was consistent with hepatic decompensation. The most frequent TEAEs consistent with hepatic decompensation were ascites (n=8), esophageal variceal hemorrhage (n=4), and hepatic encephalopathy (n=2). CONCLUSIONS
This pooled analysis in 1,066 HCV-infected patients with Child-Pugh A cirrhosis confirms the safety of OBV/PTV/r±DSV±RBV in this population. These results support the use of OBV/PTV/r±DSV±RBV in this high-priority population. Lay summary: This pooled safety analysis in 1,066 HCV-infected patients with compensated cirrhosis, receiving treatment with ombitasvir, paritaprevir, and ritonavir with or without dasabuvir, with or without ribavirin, shows that the rate of hepatic decompensation events was similar to previously reported rates in untreated patients.
中文翻译:

HCV 诱导的 Child-Pugh A 型肝硬化 2D/3D 直接作用抗病毒方案的安全性——汇总分析
背景和目的 慢性丙型肝炎病毒 (HCV) 感染的肝硬化患者是优先治疗的人群。帮助了解 Child-Pugh A 患者中 ombitasvir、paritaprevir 和利托那韦联合或不联合达沙布韦 (OBV/PTV/r±DSV) 的全口服直接作用抗病毒 (DAA) 联合方案的获益-风险概况肝硬化,我们对 AbbVie 赞助的招募 Child-Pugh A 型肝硬化患者的临床试验进行了全面审查。方法 12 项 OBV/PTV/r±利巴韦林 (RBV) 的 2-DAA 方案或 OBV/PTV/r+DSV±RBV 的 3-DAA 方案的 12 项 II 期或 III 期临床试验,其中包括 Child-Pugh A 型肝硬化患者被审查;在 2015 年 11 月 16 日之前完成治疗的患者被纳入汇总的事后安全性评估。报告了出现治疗紧急不良事件 (TEAE)、严重 TEAE 和与肝功能失代偿相一致的 TEAE 的患者数量和百分比。结果 在 1,066 名 Child-Pugh A 型肝硬化患者中,严重 TEAE 和导致研究药物停药的 TEAE 发生率分别为 5.3%(95% 置信区间 [CI]:4.1-6.8)和 2.2%(95% CI:1.4-3.2) , 分别。13 名患者(1.2%;95% CI:0.7-2.1)的 TEAE 与肝功能失代偿一致。与肝功能失代偿一致的最常见 TEAE 是腹水 (n=8)、食管静脉曲张出血 (n=4) 和肝性脑病 (n=2)。结论 这项对 1,066 名 Child-Pugh A 型肝硬化 HCV 感染患者的汇总分析证实了 OBV/PTV/r±DSV±RBV 在该人群中的安全性。这些结果支持在这个高优先级人群中使用 OBV/PTV/r±DSV±RBV。总结:这项对 1,066 名 HCV 感染的代偿性肝硬化患者进行汇总安全性分析,这些患者接受 ombitasvir、paritaprevir 和利托那韦,联合或不联合达沙布韦,联合或不联合利巴韦林,表明肝脏失代偿事件的发生率与之前报告的发生率相似在未经治疗的患者中。
更新日期:2017-10-01
中文翻译:

HCV 诱导的 Child-Pugh A 型肝硬化 2D/3D 直接作用抗病毒方案的安全性——汇总分析
背景和目的 慢性丙型肝炎病毒 (HCV) 感染的肝硬化患者是优先治疗的人群。帮助了解 Child-Pugh A 患者中 ombitasvir、paritaprevir 和利托那韦联合或不联合达沙布韦 (OBV/PTV/r±DSV) 的全口服直接作用抗病毒 (DAA) 联合方案的获益-风险概况肝硬化,我们对 AbbVie 赞助的招募 Child-Pugh A 型肝硬化患者的临床试验进行了全面审查。方法 12 项 OBV/PTV/r±利巴韦林 (RBV) 的 2-DAA 方案或 OBV/PTV/r+DSV±RBV 的 3-DAA 方案的 12 项 II 期或 III 期临床试验,其中包括 Child-Pugh A 型肝硬化患者被审查;在 2015 年 11 月 16 日之前完成治疗的患者被纳入汇总的事后安全性评估。报告了出现治疗紧急不良事件 (TEAE)、严重 TEAE 和与肝功能失代偿相一致的 TEAE 的患者数量和百分比。结果 在 1,066 名 Child-Pugh A 型肝硬化患者中,严重 TEAE 和导致研究药物停药的 TEAE 发生率分别为 5.3%(95% 置信区间 [CI]:4.1-6.8)和 2.2%(95% CI:1.4-3.2) , 分别。13 名患者(1.2%;95% CI:0.7-2.1)的 TEAE 与肝功能失代偿一致。与肝功能失代偿一致的最常见 TEAE 是腹水 (n=8)、食管静脉曲张出血 (n=4) 和肝性脑病 (n=2)。结论 这项对 1,066 名 Child-Pugh A 型肝硬化 HCV 感染患者的汇总分析证实了 OBV/PTV/r±DSV±RBV 在该人群中的安全性。这些结果支持在这个高优先级人群中使用 OBV/PTV/r±DSV±RBV。总结:这项对 1,066 名 HCV 感染的代偿性肝硬化患者进行汇总安全性分析,这些患者接受 ombitasvir、paritaprevir 和利托那韦,联合或不联合达沙布韦,联合或不联合利巴韦林,表明肝脏失代偿事件的发生率与之前报告的发生率相似在未经治疗的患者中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号