当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting the Quinoxalinedione Scaffold in the Construction of New Ligands for the Ionotropic Glutamate Receptors
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acschemneuro.7b00243 Charles S. Demmer 1 , David Rombach 1 , Na Liu 1 , Birgitte Nielsen 1 , Darryl S. Pickering 1 , Lennart Bunch 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acschemneuro.7b00243 Charles S. Demmer 1 , David Rombach 1 , Na Liu 1 , Birgitte Nielsen 1 , Darryl S. Pickering 1 , Lennart Bunch 1
Affiliation
|
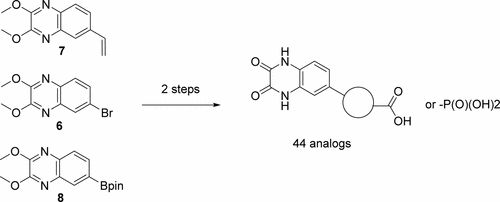
More than two decades ago, the quinoxalinedione scaffold was shown to act as an α-amino acid bioisoster. Following extensive structure–activity relationship (SAR) studies, the antagonists DNQX, CNQX, and NBQX in the ionotropic glutamate receptor field were identified. In this work, we revisit the quinoxalinedione scaffold and explore the incorporation of an acid functionality in the 6-position. The SAR studies disclose that by this strategy it was possible to tune in iGluR selectivity among the AMPA, NMDA, and KA receptors, and to some extent also obtain full receptor subtype selectivity. Highlights of the study of 44 new analogues are compound 2m being a high affinity ligand for native AMPA receptors (IC50= 0.48 μM), analogues 2e,f,h,k,v all displayed selectivity for native NMDA receptors, and compounds 2s,t,u are selective ligand for the GluK1 receptor. Most interestingly, compound 2w was shown to be a GluK3-preferring ligand with full selectivity over native AMPA, KA and NMDA receptors.
中文翻译:

复习喹喔啉二酮支架在新型配体中的离子型谷氨酸受体。
二十多年前,喹喔啉二酮支架被证明是α-氨基酸的生物等排体。经过广泛的结构-活性关系(SAR)研究,在离子型谷氨酸受体领域确定了拮抗剂DNQX,CNQX和NBQX。在这项工作中,我们将重新研究喹喔啉二酮支架,并探索在6位上引入酸官能团的方法。SAR研究表明,通过这种策略,可以调节AMPA,NMDA和KA受体之间的iGluR选择性,并且在某种程度上还可以获得完全的受体亚型选择性。研究44个新类似物的重点是化合物2m是天然AMPA受体的高亲和力配体(IC 50 = 0.48μM),类似物2e,f,h,k,v均显示出对天然NMDA受体的选择性,化合物2s,t,u是GluK1受体的选择性配体。最有趣的是,化合物2w被证明是GluK3优先配体,对天然AMPA,KA和NMDA受体具有完全选择性。
更新日期:2017-09-05
中文翻译:

复习喹喔啉二酮支架在新型配体中的离子型谷氨酸受体。
二十多年前,喹喔啉二酮支架被证明是α-氨基酸的生物等排体。经过广泛的结构-活性关系(SAR)研究,在离子型谷氨酸受体领域确定了拮抗剂DNQX,CNQX和NBQX。在这项工作中,我们将重新研究喹喔啉二酮支架,并探索在6位上引入酸官能团的方法。SAR研究表明,通过这种策略,可以调节AMPA,NMDA和KA受体之间的iGluR选择性,并且在某种程度上还可以获得完全的受体亚型选择性。研究44个新类似物的重点是化合物2m是天然AMPA受体的高亲和力配体(IC 50 = 0.48μM),类似物2e,f,h,k,v均显示出对天然NMDA受体的选择性,化合物2s,t,u是GluK1受体的选择性配体。最有趣的是,化合物2w被证明是GluK3优先配体,对天然AMPA,KA和NMDA受体具有完全选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号