Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Designs for Organoaluminum C2Al4R4 with Well-Separated Al(I) and Al(III)
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acsomega.7b00487 Ying-ying Xue 1 , Jing-jing Sui 1 , Jing Xu 2 , Yi-hong Ding 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acsomega.7b00487 Ying-ying Xue 1 , Jing-jing Sui 1 , Jing Xu 2 , Yi-hong Ding 1
Affiliation
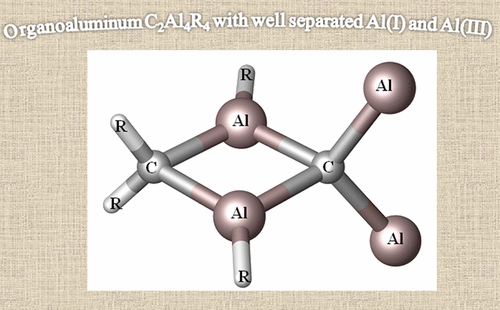
|
It is well-known that the chemistry of aluminum is dominated by Al(III) in the +3 oxidation state. Only during the past 2 decades has the chemistry of Al(I) and Al(II) been rapidly developed. However, if Al(I) and Al(III) are combined, the inherently high reactivities of Al(I) and Al(III) mostly result in their coupling with each other or interacting with surrounding elements, which easily results in significant deactivation or quenching of the desired oxidation states, as in the case of reported mixed valent Al-compounds. In this article, we report an unprecedented type of organoaluminum system, C2Al4R4 (R = H, SiH3, Si(C6H5)3, SiiPrDis2, SiMe(SitBu3)2), whose lowest-energy structure, C2Al4R4-01, contains two Al(I) and two Al(III) atoms. The global nature and bonding motif of the parent C2Al4R4-01 (R = H) were supported by an extensive global isomeric search, CBS-QB3 energy calculations, adaptive natural density partitioning, and bond order analysis. Interestingly and in sharp contrast to most organoaluminum species, C2Al4R4-01 is associated with little multicenter bonding. C2Al4R4-01 has a high feasibility of being observed either in the gas or condensed phases (with suitable substitutents). With well-separated Al(I) and Al(III), C2Al4R4-01 (with suitable substitutents) could serve as the first Al/Al frustrated Lewis pair.
中文翻译:

具有良好分离的Al(I)和Al(III)的有机铝C 2 Al 4 R 4的理论设计
众所周知,铝的化学性质以+3氧化态的Al(III)为主。仅在过去的二十年中,Al(I)和Al(II)的化学才得到快速发展。但是,如果将Al(I)和Al(III)结合使用,则Al(I)和Al(III)固有的高反应性通常会导致它们彼此偶联或与周围元素相互作用,从而很容易导致显着失活或所需氧化态的猝灭,如报道的混合价铝化合物的情况一样。在本文中,我们报告了一种前所未有的有机铝体系,即C 2 Al 4 R 4(R = H,SiH 3,Si(C 6 H 5)3,Si iPrDis 2,森达(SI吨卜3)2),其能量最低的结构,C 2的Al 4 - [R 4 - 01,包含两个铝(I)和两个铝(III)原子。父C的全球性和粘合基序2的Al 4 - [R 4 - 01(R = H)是由广泛的全球搜索异构体支持,CBS-QB3能量计算,自适应自然密度分区和键级分析。有趣的是与形成鲜明对比,大多数有机铝物种,C 2的Al 4 - [R 4 - 01与很少的多中心绑定相关联。c ^ 2的Al 4 - [R 4 - 01具有气体或冷凝相在任一被观察的高可行性(具有适当的取代基)。与分离良好的铝(I)和Al(III),C 2的Al 4 - [R 4 - 01(具有适当的取代基)可以作为第一Al / Al的受挫刘易斯对。
更新日期:2017-09-05
中文翻译:

具有良好分离的Al(I)和Al(III)的有机铝C 2 Al 4 R 4的理论设计
众所周知,铝的化学性质以+3氧化态的Al(III)为主。仅在过去的二十年中,Al(I)和Al(II)的化学才得到快速发展。但是,如果将Al(I)和Al(III)结合使用,则Al(I)和Al(III)固有的高反应性通常会导致它们彼此偶联或与周围元素相互作用,从而很容易导致显着失活或所需氧化态的猝灭,如报道的混合价铝化合物的情况一样。在本文中,我们报告了一种前所未有的有机铝体系,即C 2 Al 4 R 4(R = H,SiH 3,Si(C 6 H 5)3,Si iPrDis 2,森达(SI吨卜3)2),其能量最低的结构,C 2的Al 4 - [R 4 - 01,包含两个铝(I)和两个铝(III)原子。父C的全球性和粘合基序2的Al 4 - [R 4 - 01(R = H)是由广泛的全球搜索异构体支持,CBS-QB3能量计算,自适应自然密度分区和键级分析。有趣的是与形成鲜明对比,大多数有机铝物种,C 2的Al 4 - [R 4 - 01与很少的多中心绑定相关联。c ^ 2的Al 4 - [R 4 - 01具有气体或冷凝相在任一被观察的高可行性(具有适当的取代基)。与分离良好的铝(I)和Al(III),C 2的Al 4 - [R 4 - 01(具有适当的取代基)可以作为第一Al / Al的受挫刘易斯对。











































 京公网安备 11010802027423号
京公网安备 11010802027423号