Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.mcat.2017.08.017 Yan Li , Tingting Liu , Changhai Liang
|
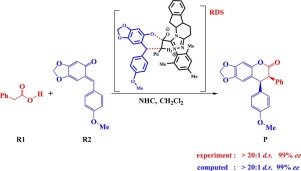
The mechanisms and stereoselectivities in N-heterocyclic carbene-catalyzed [4 + 2] cycloadditions of phenylacetic acid and o-quinone methide have been studied by the use of density functional theory (DFT) calculations. Various possible reaction pathways were located and compared. The most energy favorable pathway can be characterized by four stages: the formation of intermediate IM2 via the nucleophilic attack of catalyst to phenylacetic acid (stage I); deprotonation of IM2 to generate the NHC-bounded enolate intermediate IM4 (stage II); addition of IM4 to R2 to form the six-membered ring intermediate IM5 (stage III) and elimination of catalyst leading to the RS-configuration product P(RS) (stage IV). For stage II, both direct deprotonation and base-mediated deprotonation were examined, DFT calculations indicate that traces of base are essential for the deprotonation process. The [4 + 2] cycloaddition step (stage III) is found to be the rate- and stereoselectivity-determining step with an overall free energy barrier of 16.6 kcal/mol. The predicted high cis-diastereoselectivities and enantioselectivities for the [4 + 2] annulation reaction are in good agreement with the experimental observations. The present study should be useful to the development of this kind reactions in the future.
中文翻译:

NHC催化[4 + 2]苯乙酸与邻醌甲基甲烷的环加成反应的机理和立体选择性:计算研究
通过使用密度泛函理论(DFT)计算,研究了N-杂环卡宾催化的[4 + 2]苯乙酸和邻醌甲基化物的环加成反应的机理和立体选择性。找到并比较了各种可能的反应途径。最节能的途径可分为四个阶段:通过催化剂对苯乙酸的亲核攻击形成中间体IM2(阶段I);IM2去质子化以生成NHC结合的烯酸酯中间体IM4(阶段II); 在R2中添加IM4以形成六元环中间体IM5(第III阶段))并消除产生RS构型产物P(RS)的催化剂(阶段IV)。对于第二阶段,直接去质子化和碱介导的去质子化都进行了检查,DFT计算表明碱的痕迹对于去质子化过程至关重要。发现[4 + 2]环加成步骤(阶段III)是速率和立体选择性的确定步骤,总自由能垒为16.6 kcal / mol。预测的[4 + 2]环化反应的高顺式-非对映选择性和对映体选择性与实验观察结果非常吻合。本研究对将来这种反应的发展将是有用的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号