Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.jconrel.2017.09.006 Aniket S. Wadajkar , Jimena G. Dancy , Nathan B. Roberts , Nina P. Connolly , Dudley K. Strickland , Jeffrey A. Winkles , Graeme F. Woodworth , Anthony J. Kim
|
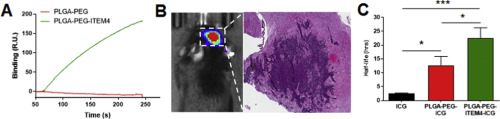
The most common and deadly form of primary brain cancer, glioblastoma (GBM), is characterized by significant intratumoral heterogeneity, microvascular proliferation, immune system suppression, and brain tissue invasion. Delivering effective and sustained treatments to the invasive GBM cells intermixed with functioning neural elements is a major goal of advanced therapeutic systems for brain cancer. Previously, we investigated the nanoparticle characteristics that enable targeting of invasive GBM cells. This revealed the importance of minimizing non-specific binding within the relatively adhesive, ‘sticky’ microenvironment of the brain and brain tumors in particular. We refer to such nanoformulations with decreased non-specific adhesivity and receptor targeting as ‘DART’ therapeutics. In this work, we applied this information toward the design and characterization of biodegradable nanocarriers, and in vivo testing in orthotopic experimental gliomas. We formulated particulate nanocarriers using poly(lactic-co-glycolic acid) (PLGA) and PLGA-polyethylene glycol (PLGA-PEG) polymers to generate sub-100 nm nanoparticles with minimal binding to extracellular brain components and strong binding to the Fn14 receptor – an upregulated, conserved component in invasive GBM. Multiple particle tracking in brain tissue slices and in vivo testing in orthotopic murine malignant glioma revealed preserved nanoparticle diffusivity and increased uptake in brain tumor cells. These combined characteristics also resulted in longer retention of the DART nanoparticles within the orthotopic tumors compared to non-targeted versions. Taken together, these results and nanoparticle design considerations offer promising new methods to optimize therapeutic nanocarriers for improving drug delivery and treatment for invasive brain tumors.
中文翻译:

降低的非特异性粘附性,受体靶向(DART)纳米颗粒在浸润性神经胶质瘤中表现出改善的分散性,细胞摄取和肿瘤保留
原发性脑癌最常见和致命的形式是胶质母细胞瘤(GBM),其特征是明显的肿瘤内异质性,微血管增生,免疫系统抑制和脑组织浸润。对与功能性神经元混合在一起的浸润性GBM细胞进行有效和持续的治疗,是先进的脑癌治疗系统的主要目标。以前,我们研究了能够靶向侵袭性GBM细胞的纳米颗粒特征。这揭示了最小化相对粘着的,特别是脑部和脑部肿瘤的“粘性”微环境内的非特异性结合的重要性。我们将这种具有非特异性粘附力和受体靶向降低的纳米制剂称为“ DART”疗法。在这项工作中,原位实验性神经胶质瘤的体内测试。我们配制使用聚(乳酸-微粒纳米载体共-glycolic乙酸)(PLGA)和PLGA-聚乙二醇(PLGA-PEG)聚合物,以生成低于100nm的纳米颗粒具有最小结合胞外脑组件和强结合FN14受体-侵入性GBM中上调的保守成分。脑组织切片和体内的多粒子跟踪在原位鼠恶性神经胶质瘤中进行的测试显示,纳米颗粒的扩散性得以保留,脑肿瘤细胞的摄取也有所增加。与非靶向形式相比,这些组合特征还导致DART纳米粒子在原位肿瘤内的保留时间更长。综上所述,这些结果和纳米颗粒设计的考虑因素提供了有前途的新方法,可以优化治疗性纳米载体,从而改善侵袭性脑肿瘤的药物递送和治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号