Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.apcata.2017.09.002 Shan Ping Liu , Ming Zhao , Yong Fu Zhu , Wang Gao , Qing Jiang
|
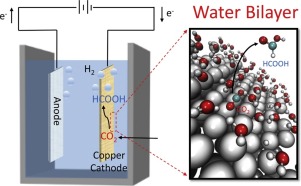
Solid–liquid interface, which is the location that electrochemical reaction occurs, is essential for CO2 reduction. However, different theoretical calculations often use different models of solid–liquid interface and predict conflicting mechanisms, e.g. for the formation of HCOOH. To address this issue, we adopt different structures of solid–liquid interface to mimic the reaction circumstance using density functional theory calculations. We find that the reaction barriers and energies are sensitive to the reaction circumstance, such as the state of water molecules, the number of water molecules, and the available surface sites, whereas the selectivity of CO2 reduction can be hardly changed once a water bilayer network on Cu surface is used. In particular, a solid–liquid interface built from 4-6-4 water rings (5H2O/1H) can present an appropriate description of the solvent, predicting the formation pathways of HCOOH in reasonable agreement with the experiments. These results confirm the role of water bilayer networks in describing solid–liquid interface in the electrocatalysis, which serves as an important basis for the future studies.
中文翻译:

在铜上将CO 2电还原为甲酸:水双层在电化学界面建模中的作用
固液界面是发生电化学反应的位置,对于还原CO 2至关重要。但是,不同的理论计算通常使用固液界面的不同模型并预测相互矛盾的机制,例如形成HCOOH的方式。为了解决这个问题,我们采用密度泛函理论计算采用不同的固液界面结构来模拟反应情况。我们发现反应势垒和能量对反应情况敏感,例如水分子的状态,水分子的数量和可用的表面位点,而CO 2的选择性一旦在铜表面上使用水双层网络,还原几乎无法改变。特别是,由4-6-4个水环(5H 2 O / 1H)建立的固-液界面可以恰当地描述溶剂,并与实验合理地预测HCOOH的形成途径。这些结果证实了水双层网络在描述电催化中的固液界面方面的作用,这为将来的研究提供了重要的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号