当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.apcatb.2017.09.006 Xuqiang Hao , Yicong Wang , Jun Zhou , Zhiwei Cui , Ying Wang , Zhigang Zou
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.apcatb.2017.09.006 Xuqiang Hao , Yicong Wang , Jun Zhou , Zhiwei Cui , Ying Wang , Zhigang Zou
|
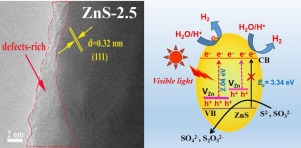
Zinc sulfide is a superior photocatalyst for H2 evolution, whereas the wide bandgap restricts its performance to only UV region. In this work, zinc vacancy (VZn) defects are successfully introduced into ZnS via adding sodium sulfide as sulfur source during the hydrothermal reaction. The defective ZnS with different amount of zinc vacancies were employed as catalysts for the examination of vacancy-dependent catalytic activity toward photocatalytic hydrogen evolution under visible light irradiation. Fluorescence emission spectra and XPS results confirm that existence of abundant zinc vacancies on ZnS. These zinc vacancies exhibit remarkable effects on modifying the electronic structure of ZnS as shown in UV−visible absorption spectra and Mott−Schottky plots. Zinc vacancies can raise valence band (VB) position that weaken the oxidative capacity of the holes to protect Zn-deficient ZnS from photocorrsion. And electrochemical and photo-electrochemical experiments also demonstrate that the charge separation and the electrons transfer are more efficient with the introduction of the Zn vacancies in ZnS. The zinc-deficient ZnS-2.5 with optimum amount of Zn vacancies shows superior photocatalytic activity for H2 evolution that reaches 337.71± 3.72 μmol h−1g−1 under visible-light irradiation and also exhibits a much higher photostability. The intrinsic modify by self-defects might be a potential strategy for design novel photocatalysts with photocorrosion stability and visible-light activity in photocatalysis proton reduction.
中文翻译:

锌空位促进了ZnS的光催化活性和光稳定性,从而有效地驱动了可见光驱动的氢气释放
硫化锌是H 2析出的优良光催化剂,而宽带隙将其性能限制在仅UV区。在这项工作中,锌空位(V Zn)缺陷已通过在水热反应中加入硫化钠作为硫源。使用具有不同锌空位数量的有缺陷的ZnS作为催化剂,以研究可见光照射下空位对光催化氢释放的催化活性。荧光发射光谱和XPS结果证实在ZnS上存在大量锌空位。这些锌空位在改变ZnS的电子结构方面表现出显着的影响,如UV-可见吸收光谱和Mott-Schottky图所示。锌空位可以提高价带(VB)的位置,从而削弱空穴的氧化能力,从而保护缺锌的ZnS免受光腐蚀。电化学和光电化学实验还表明,随着ZnS中Zn空位的引入,电荷分离和电子转移更加有效。具有最佳锌空位量的缺锌ZnS-2.5显示出对H的优异光催化活性在可见光照射下,2演化达到337.71±3.72μmolh -1 g -1,并且还表现出更高的光稳定性。通过自缺陷进行的内在修饰可能是设计具有光腐蚀稳定性和可见光活性的新型光催化剂在光催化质子还原中的潜在策略。
更新日期:2017-09-06
中文翻译:

锌空位促进了ZnS的光催化活性和光稳定性,从而有效地驱动了可见光驱动的氢气释放
硫化锌是H 2析出的优良光催化剂,而宽带隙将其性能限制在仅UV区。在这项工作中,锌空位(V Zn)缺陷已通过在水热反应中加入硫化钠作为硫源。使用具有不同锌空位数量的有缺陷的ZnS作为催化剂,以研究可见光照射下空位对光催化氢释放的催化活性。荧光发射光谱和XPS结果证实在ZnS上存在大量锌空位。这些锌空位在改变ZnS的电子结构方面表现出显着的影响,如UV-可见吸收光谱和Mott-Schottky图所示。锌空位可以提高价带(VB)的位置,从而削弱空穴的氧化能力,从而保护缺锌的ZnS免受光腐蚀。电化学和光电化学实验还表明,随着ZnS中Zn空位的引入,电荷分离和电子转移更加有效。具有最佳锌空位量的缺锌ZnS-2.5显示出对H的优异光催化活性在可见光照射下,2演化达到337.71±3.72μmolh -1 g -1,并且还表现出更高的光稳定性。通过自缺陷进行的内在修饰可能是设计具有光腐蚀稳定性和可见光活性的新型光催化剂在光催化质子还原中的潜在策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号