Talanta ( IF 5.6 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.talanta.2017.08.105 Brittany L. Grimm , Libby A. Stern , Alexander J. Lowe
|
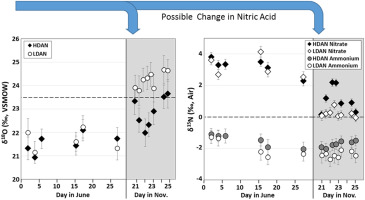
Ammonium nitrate (AN) based fertilizers are inexpensive and easily obtained, characteristics that often lead to their use in homemade explosive devices. The stable nitrogen and oxygen isotope ratios (15N/14N and 18O/16O, expressed as δ15N and δ18O) of AN have the potential to aid in forensic investigations by providing supplemental properties for sample-to-sample comparison in materials which are otherwise chemically identical. The forensic utility of stable isotope analyses depends on demonstrated variation between different sources and minimal variation within a source. To test the variability within a single manufacturer (here considered a source), a total of 26 samples representing two production time periods and two product lines were analyzed for bulk δ15N and δ18O. Additionally, because AN is known to have a modest isotopic range, a potassium nitrate precipitation method was developed to separate the component ions (NO3- and NH4+) for individual δ15N analysis and increased discriminatory power. The average δ15N and δ18O of bulk AN (− 0.10‰ and + 22.8‰, respectively) is similar to the isotopic signature of atmospheric N2 and O2, the starting reactants in AN production. The bulk δ15N, δ18O, and NO3- δ15N show average values from both product lines that differ by 1.5‰, 2.0‰, and 2.6‰, respectively, between the production periods of June and November 2015. Conversely, the NH4+ δ15N remained relatively consistent over time. Furthermore, whereas samples in the two product lines produced on the same day in June are isotopically similar, there are isotopic differences between samples in the two product lines manufactured within 6 h of each other in November. The observed variability could be useful in comparing AN from two or more bombs, or a bomb and a stash of AN in a suspect's possession, but the observed lot-to-lot differences within one manufacturer could complicate attribution efforts. In contrast, the NH4+ δ15N values, which appear to be the most consistent over time within this factory, need to be further explored as a potentially reliable signal.
中文翻译:

硝酸铵及其离析离子的氮氧同位素比率时间序列的法医学应用
硝酸铵(AN)基肥料价格便宜,易于获得,这种特性经常导致其在自制爆炸装置中的使用。稳定的氮和氧的同位素比例(15 N / 14 N和18 O / 16 O,表示为δ 15 N和δ 18O)的AN具有为法医研究提供帮助的潜力,因为它提供了化学性质相同的材料中样品间比较的补充特性。稳定同位素分析的法证实用性取决于已证明的不同来源之间的差异以及来源内的最小差异。测试单个制造商(这里考虑的源极)内的可变性,共表示两个生产时间段和两条产品线26个的样品进行分析的散装δ 15 N和δ 18 O.另外,由于AN已知具有一个适度同位素范围,硝酸钾沉淀法的开发是为了在成分分离离子(NO 3 -和NH 4 +),用于个人δ 15 Ñ分析和增加的区分能力。平均δ 15 N和δ 18散装AN的O( - 0.10‰和+ 22.8‰,分别)是类似于大气氮的同位素特征2和O 2,在AN生产起始反应物。散装δ 15 N,δ 18 O,和NO 3 - δ 15 Ñ分别示出了平均值与由1.5两者不同的产品线‰,2.0‰和2.6‰,六月和十一月2015相反的生产周期之间时,NH 4 + δ 15随着时间的流逝,N保持相对稳定。此外,尽管在6月同一天生产的两条生产线中的样品在同位素上相似,但在11月彼此相距6小时之内生产的两条生产线中的样品之间存在同位素差异。观察到的变异性可用于比较来自两个或更多炸弹的炸弹,或者比较犯罪嫌疑人拥有的炸弹和炸弹的藏身之处,但观察到的一个制造商之间批次间的差异可能会使归因工作复杂化。相反,NH 4 + δ 15 N个值,这似乎是此工厂中最一致的随时间的,需要进一步探讨作为潜在的可靠的信号。











































 京公网安备 11010802027423号
京公网安备 11010802027423号