当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction between Pyridine and CH3NH3PbI3: Surface-Confined Reaction or Bulk Transformation?
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acs.jpca.7b05423 Xiaozhou Yu 1 , Haoming Yan 1 , Qing Peng 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acs.jpca.7b05423 Xiaozhou Yu 1 , Haoming Yan 1 , Qing Peng 1
Affiliation
|
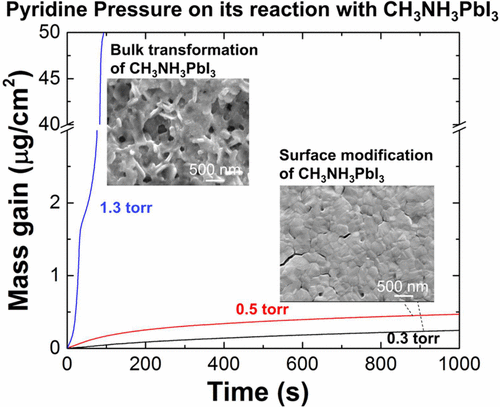
Pyridine molecules have been used to passivate surface Pb2+ sites of CH3NH3PbI3, to recrystallize CH3NH3PbI3, and to bleach CH3NH3PbI3. However, these results contradict each other, as recrystallization and optical-bleach require transformation of bulk CH3NH3PbI3, but surface passivation demands the confinement of the reaction at the surface region. The underlying mechanism for these seemly contradicting results is not yet understood. In this paper, we show, at 25 °C, partial pressure of pyridine vapor is a determining factor for its reaction behaviors with CH3NH3PbI3: one can modify the surface region of CH3NH3PbI3 by using pyridine vapor of pressure 1.15 torr or lower but can transform the whole bulk CH3NH3PbI3 film with a pyridine vapor of 1.3 torr or higher. Our result is the first demonstration that the reaction modes, i.e., surface-confined reaction and bulk transformation, are very sensitive to the partial pressure of under-saturated pyridine vapor. Despite the different reaction behaviors, it is interesting that in all pressure ranges, pyridinium ion is a main product from the reaction between pyridine and CH3NH3PbI3. The bulk transformation is due to the formation of a liquid-like film, which increases the mobility of species to catalyze the reaction between pyridine and CH3NH3PbI3. It is important to note 1.3 torr is much smaller than the saturated vapor pressure of pyridine (20 torr at 25 °C). These findings provide a guidance in applying pyridine and other amines to functionalize and transform CH3NH3PbI3 and other hybrid halide perovskites. It also highlights the critical role of fundamental studies in controllably modifying CH3NH3PbI3.
中文翻译:

吡啶与CH 3 NH 3 PbI 3之间的反应:表面受限反应还是本体转变?
吡啶分子已被用于钝化表面的Pb 2+ CH的位点3 NH 3碘化铅3,再结晶CH 3 NH 3碘化铅3,和漂白CH 3 NH 3碘化铅3。但是,这些结果相互矛盾,因为重结晶和光漂白剂需要转化大量的CH 3 NH 3 PbI 3。但是,表面钝化要求将反应限制在表面区域。这些看似矛盾的结果的潜在机制尚不清楚。本文表明,在25°C时,吡啶蒸气的分压是其与CH 3 NH 3 PbI 3的反应行为的决定因素:人们可以通过使用吡啶蒸气来修饰CH 3 NH 3 PbI 3的表面区域。的压力为1.15托或更低,但可以转变整个体积的CH 3 NH 3 PbI 3吡啶蒸气为1.3托或更高的薄膜。我们的结果是第一个证明反应模式,即表面受限反应和本体转化,对欠饱和吡啶蒸气的分压非常敏感。尽管反应行为不同,但有趣的是,在所有压力范围内,吡啶鎓离子是吡啶与CH 3 NH 3 PbI 3之间反应的主要产物。整体转化是由于形成了液体状薄膜,该薄膜增加了物种的迁移率,以催化吡啶与CH 3 NH 3 PbI 3之间的反应。。重要的是要注意1.3托要比吡啶的饱和蒸气压(在25°C时为20托)小得多。这些发现为应用吡啶和其他胺官能化和转化CH 3 NH 3 PbI 3和其他杂化卤化物钙钛矿提供了指导。它还强调了基础研究在可控制地修饰CH 3 NH 3 PbI 3中的关键作用。
更新日期:2017-09-04
中文翻译:

吡啶与CH 3 NH 3 PbI 3之间的反应:表面受限反应还是本体转变?
吡啶分子已被用于钝化表面的Pb 2+ CH的位点3 NH 3碘化铅3,再结晶CH 3 NH 3碘化铅3,和漂白CH 3 NH 3碘化铅3。但是,这些结果相互矛盾,因为重结晶和光漂白剂需要转化大量的CH 3 NH 3 PbI 3。但是,表面钝化要求将反应限制在表面区域。这些看似矛盾的结果的潜在机制尚不清楚。本文表明,在25°C时,吡啶蒸气的分压是其与CH 3 NH 3 PbI 3的反应行为的决定因素:人们可以通过使用吡啶蒸气来修饰CH 3 NH 3 PbI 3的表面区域。的压力为1.15托或更低,但可以转变整个体积的CH 3 NH 3 PbI 3吡啶蒸气为1.3托或更高的薄膜。我们的结果是第一个证明反应模式,即表面受限反应和本体转化,对欠饱和吡啶蒸气的分压非常敏感。尽管反应行为不同,但有趣的是,在所有压力范围内,吡啶鎓离子是吡啶与CH 3 NH 3 PbI 3之间反应的主要产物。整体转化是由于形成了液体状薄膜,该薄膜增加了物种的迁移率,以催化吡啶与CH 3 NH 3 PbI 3之间的反应。。重要的是要注意1.3托要比吡啶的饱和蒸气压(在25°C时为20托)小得多。这些发现为应用吡啶和其他胺官能化和转化CH 3 NH 3 PbI 3和其他杂化卤化物钙钛矿提供了指导。它还强调了基础研究在可控制地修饰CH 3 NH 3 PbI 3中的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号