当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cis/Trans Isomerization in Secondary Amides: Reaction Paths, Nitrogen Inversion, and Relevance to Peptidic Systems
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.jpca.7b05584 Balmukund S. Thakkar 1 , John-Sigurd M. Svendsen 1 , Richard A. Engh 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.jpca.7b05584 Balmukund S. Thakkar 1 , John-Sigurd M. Svendsen 1 , Richard A. Engh 1
Affiliation
|
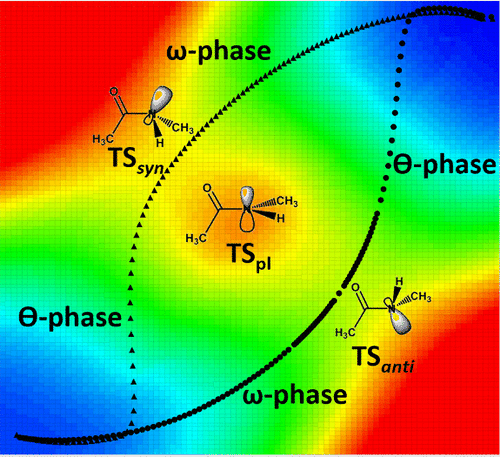
Cis/trans isomerization of 2°-amide bonds is a key step in a wide range of important processes. Here we present a theoretical assessment of cis/trans isomerization of 2°-amide bonds using B3LYP density functional methods, describing two reaction paths and corresponding geometry changes during isomerization of N-methylacetamide (NMA) and glycylglycine methyl ester (GGMe). The isomerization begins via a common path, as the extended π-bonding of the amide bond maintains approximate planarity of the O–C–N–H dihedral angle, with only gradually increasing pyramidalization of the nitrogen atom, until a bifurcation point is reached. Both subsequent paths comprise two phases, an “ω phase” (characterized by a major change in C–C–N–C dihedral) and a “θ phase” (characterized by major change in O–C–N–H dihedral), with two distinct transition states. The θ phase involves inversion of the pyramidal amide-nitrogen geometry. Both reaction paths converge at another bifurcation point near the opposite geometry. Studies on the larger GGMe show in addition that the multiple additional rotamers do not change the qualitative properties of the isomerization, but do affect the energies of the differing transition states. These detailed results provide significant new insights into cis/trans isomerization paths in 2°-amides, and serve as a basis for theoretical studies on larger peptidic systems.
中文翻译:

仲酰胺中的顺式/反式异构化:反应路径,氮转化和与肽系统的相关性
2°-酰胺键的顺/反异构是许多重要过程中的关键步骤。在这里,我们介绍使用B3LYP密度泛函方法对2°-酰胺键进行顺式/反式异构化的理论评估,描述了N异构化过程中的两个反应路径和相应的几何变化-甲基乙酰胺(NMA)和甘氨酰甘氨酸甲酯(GGMe)。异构化是通过一条共同的路径开始的,因为酰胺键的扩展π键保持了O–C–N–H二面角的近似平面性,仅逐渐增加了氮原子的锥体化作用,直到达到分叉点为止。随后的两个路径都包含两个阶段,一个“ω相”(由CC–N–C二面体的主要变化表征)和一个“θ相”(由OC–N–H二面体的主要变化表征),具有两个不同的过渡态 θ相涉及锥体酰胺-氮几何形状的反转。两个反应路径在相反的几何形状附近会聚在另一个分叉点。对更大的GGMe的研究还表明,多个其他的旋转异构体不会改变异构化的定性性质,但确实会影响不同过渡态的能量。这些详细的结果提供了对2°-酰胺中顺式/反式异构化途径的重要新见解,并为较大肽系统的理论研究奠定了基础。
更新日期:2017-09-04
中文翻译:

仲酰胺中的顺式/反式异构化:反应路径,氮转化和与肽系统的相关性
2°-酰胺键的顺/反异构是许多重要过程中的关键步骤。在这里,我们介绍使用B3LYP密度泛函方法对2°-酰胺键进行顺式/反式异构化的理论评估,描述了N异构化过程中的两个反应路径和相应的几何变化-甲基乙酰胺(NMA)和甘氨酰甘氨酸甲酯(GGMe)。异构化是通过一条共同的路径开始的,因为酰胺键的扩展π键保持了O–C–N–H二面角的近似平面性,仅逐渐增加了氮原子的锥体化作用,直到达到分叉点为止。随后的两个路径都包含两个阶段,一个“ω相”(由CC–N–C二面体的主要变化表征)和一个“θ相”(由OC–N–H二面体的主要变化表征),具有两个不同的过渡态 θ相涉及锥体酰胺-氮几何形状的反转。两个反应路径在相反的几何形状附近会聚在另一个分叉点。对更大的GGMe的研究还表明,多个其他的旋转异构体不会改变异构化的定性性质,但确实会影响不同过渡态的能量。这些详细的结果提供了对2°-酰胺中顺式/反式异构化途径的重要新见解,并为较大肽系统的理论研究奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号