当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rethinking the Dehydrogenative Amide Synthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acscatal.7b02415 Dmitry G. Gusev 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acscatal.7b02415 Dmitry G. Gusev 1
Affiliation
|
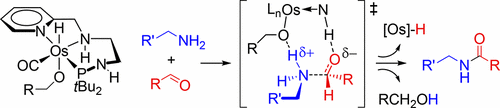
This paper is concerned with the mechanism of the catalytic dehydrogenative coupling of primary alcohols with amines; it addresses the question on what happens to the aldehyde produced in the catalytic solution upon dehydrogenation of an alcohol substrate. Here we demonstrate a rapid catalytic reaction of acetaldehyde with primary amines, leading to acetamides. The facile amide bond formation is a low-energy, outer-sphere catalytic process elucidated with the help of DFT calculations. Overall, the dehydrogenative amide synthesis comprises two metal-catalyzed cycles: the first producing the aldehyde and H2, and the second where the hemiaminal is formed and is dehydrogenated. The results call into question the existing mechanistic ideas (reviewed by Li and Hall) that invoke the uncatalyzed formation of a free hemiaminal intermediate and assume that the hemiaminal dehydrogenation requires a 16-electron catalyst.
中文翻译:

对脱氢酰胺合成的反思
本文涉及伯醇与胺的催化脱氢偶联机理。它解决了醇底物脱氢后催化溶液中产生的醛发生了什么问题。在这里,我们证明了乙醛与伯胺的快速催化反应,生成乙酰胺。简便的酰胺键形成是一种低能量的外层催化过程,借助DFT计算得以阐明。总的来说,脱氢酰胺的合成包括两个金属催化的循环:第一个产生醛和H 2。,第二个是形成血胺醛并脱氢的地方。结果令人质疑现有的机制思想(由Li和Hall审查),这些思想援引了游离的半缩醛中间体的未催化形成,并假设半缩醛脱氢需要16电子催化剂。
更新日期:2017-09-04
中文翻译:

对脱氢酰胺合成的反思
本文涉及伯醇与胺的催化脱氢偶联机理。它解决了醇底物脱氢后催化溶液中产生的醛发生了什么问题。在这里,我们证明了乙醛与伯胺的快速催化反应,生成乙酰胺。简便的酰胺键形成是一种低能量的外层催化过程,借助DFT计算得以阐明。总的来说,脱氢酰胺的合成包括两个金属催化的循环:第一个产生醛和H 2。,第二个是形成血胺醛并脱氢的地方。结果令人质疑现有的机制思想(由Li和Hall审查),这些思想援引了游离的半缩醛中间体的未催化形成,并假设半缩醛脱氢需要16电子催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号