当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Conjugate Hydrocyanation of α,β-Unsaturated N-Acylpyrroles Catalyzed by Chiral Lithium(I) Phosphoryl Phenoxide
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acscatal.7b02551 Manabu Hatano 1 , Katsuya Yamakawa 1 , Kazuaki Ishihara 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acscatal.7b02551 Manabu Hatano 1 , Katsuya Yamakawa 1 , Kazuaki Ishihara 1
Affiliation
|
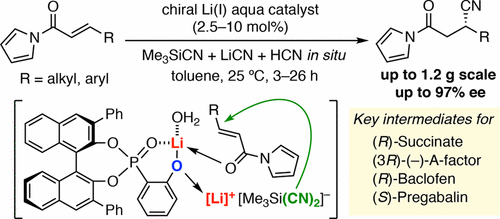
Enantioselective conjugate hydrocyanation of α,β-unsaturated N-acylpyrroles with the combined use of Me3SiCN, LiCN, and HCN has been developed in the presence of a chiral lithium(I) phosphoryl phenoxide catalyst. This reaction is useful for a variety of N-acylpyrroles, including previously unreported substrates, such as heteroaryl and halogen-substituted N-cinnamoylpyrroles. A gram-scale reaction and subsequent transformations to a (R)-succinate, (S)-paraconic acid, and (R)-baclofen demonstrate an entry for the practical synthesis of optically active β-substituted γ-aminobutyric acids (GABA).
中文翻译:

手性磷酸锂(I)催化α,β-不饱和N-酰基吡咯的对映选择性共轭氢氰化
在手性锂(I)磷酰基苯酚催化剂的存在下,开发了结合使用Me 3 SiCN,LiCN和HCN的α,β-不饱和N-酰基吡咯的对映选择性共轭氢氰化方法。该反应可用于多种N-酰基吡咯,包括先前未报告的底物,例如杂芳基和卤素取代的N-肉桂酰基吡咯。克级反应和随后的向(R)-琥珀酸酯,(S)-对锥酸和(R)-baclofen的转化证明了光学合成β-取代的γ-氨基丁酸(GABA)的实用合成方法。
更新日期:2017-09-04
中文翻译:

手性磷酸锂(I)催化α,β-不饱和N-酰基吡咯的对映选择性共轭氢氰化
在手性锂(I)磷酰基苯酚催化剂的存在下,开发了结合使用Me 3 SiCN,LiCN和HCN的α,β-不饱和N-酰基吡咯的对映选择性共轭氢氰化方法。该反应可用于多种N-酰基吡咯,包括先前未报告的底物,例如杂芳基和卤素取代的N-肉桂酰基吡咯。克级反应和随后的向(R)-琥珀酸酯,(S)-对锥酸和(R)-baclofen的转化证明了光学合成β-取代的γ-氨基丁酸(GABA)的实用合成方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号