Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-08-31 , DOI: 10.1016/j.bmc.2017.08.008 Katharina Gloria Hugentobler , Michael Müller
|
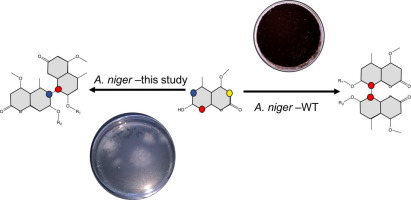
Regio- and stereoselective phenol coupling is difficult to achieve using synthetic strategies. However, in nature, cytochrome P450 enzyme-mediated routes are employed to achieve complete axial stereo- and regiocontrol in the biosynthesis of compounds with potent bioactivity. Here, we report a synthetic biology approach whereby the bicoumarin metabolic pathway in Aspergillus niger was specifically tailored towards the formation of new coupling products. This strategy represents a manipulation of the bicoumarin pathway in A. niger via interchange of the phenol-coupling biocatalyst and could be applied to other components of the pathway to access a variety of atropisomeric natural product derivatives.
中文翻译:

迈向具有联芳基轴的半合成天然化合物:黑曲霉中的氧化苯酚偶联
使用合成策略很难实现区域和立体选择性苯酚偶联。然而,实际上,在具有强生物活性的化合物的生物合成中,采用细胞色素P450酶介导的途径来实现完全的轴向立体和区域控制。在这里,我们报告了一种合成生物学方法,其中黑曲霉中的双香豆素代谢途径专门针对形成新的偶联产物而量身定制。该策略代表通过交换酚偶联生物催化剂对黑曲霉中双香豆素途径的操纵,并且可以应用于该途径的其他组分以访问各种阻转异构体天然产物衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号