Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-04 , DOI: 10.1016/j.tet.2017.08.053 Christian E. Madu , H.V. Rasika Dias , Carl J. Lovely
|
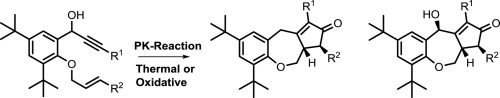
The application of the intramolecular Pauson-Khand reaction of 1,n-enynes provides a convenient method for the construction of polycyclic frameworks but this process has largely been limited to the formation of 5,5- and 5,6-fused ring systems. In this report, we describe the application of the Pauson-Khand cyclization to 1,8-enynes embedded in an aromatic ring system wherein it is determined that the presence of steric buttresses in the form of t-butyl groups facilitates the cycloaddition. These reactions proceed in good yields with either thermal or oxidative activation and in the former case, the diastereoselectivities are high. An investigation of the tolerance of this cycloaddition to substitution around the 1,8-enyne demonstrates that only 2,2-disubstitution does not result in productive cyclization. Cycloadditions with hydroxyl groups at the propargylic position while leading to fused rings are compromised by side reactions leading to reduction and in some cases tautomerization. However, these byproducts are easily minimized through conversion of the hydroxyl group to the corresponding silyl ether.
中文翻译:

苄烯炔的Pauson-Khand反应中的立体支撑
1,n-烯炔的分子内Pauson-Khand反应的应用为构建多环骨架提供了一种方便的方法,但该过程在很大程度上限于5,5-和5,6-稠合环体系的形成。在本报告中,我们描述了Pauson-Khand环化对嵌入芳香环系统中的1,8-烯炔的应用,其中确定存在以t形式存在的空间扶壁丁基有助于环加成。这些反应通过热活化或氧化活化都能以高收率进行,在前一种情况下,非对映选择性很高。对这种环加成反应对1,8-烯炔的耐受性的研究表明,只有2,2-二取代不会导致生产性环化。在导致稠合环的同时在炔丙基位置具有羟基的环加成反应会受到副反应的损害,从而导致还原反应和某些情况下的互变异构。然而,通过羟基转化为相应的甲硅烷基醚,可以容易地使这些副产物最小化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号