当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring hydroamination-cycloaddition-fragmentation sequences to access polycyclicguanidines and vinyl-2-aminoimidazoles
Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-01 , DOI: 10.1016/j.tet.2017.08.052 Ki-Hyeok Kwon , Anne V. Edwards , Miao Yang , Ryan E. Looper
中文翻译:

探索加氢胺化-环加成-断裂序列以获取多环胍和乙烯基-2-氨基咪唑
更新日期:2017-09-01
Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-01 , DOI: 10.1016/j.tet.2017.08.052 Ki-Hyeok Kwon , Anne V. Edwards , Miao Yang , Ryan E. Looper
|
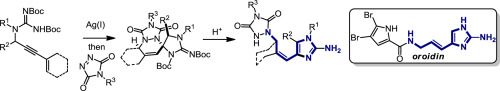
The intramolecular hydroamination of a guanidine on an eneyne unit affords a guanidine-substituted diene capable of reacting with dienophiles. These substrates undergo [4+2]-cycloaddition reactions to generate a series of complex cyclic- and spirocyclic-guanidines. Select substrates can further undergo a ring opening-elimination cascade that ultimately reveals a vinyl-2-aminoimidazole. As such this cascade reaction may find application in the synthesis of oroidin-type natural products and their analogues.
中文翻译:

探索加氢胺化-环加成-断裂序列以获取多环胍和乙烯基-2-氨基咪唑
在亚炔单元上的胍的分子内加氢胺化提供了能够与亲二烯体反应的胍取代的二烯。这些底物经历[4 + 2]-环加成反应,生成一系列复杂的环状和螺环胍。选定的底物可以进一步经历开环消除级联反应,最终显示出乙烯基-2-氨基咪唑。因此,该级联反应可用于合成麦冬蛋白型天然产物及其类似物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号