Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-09-01 , DOI: 10.1016/j.apcatb.2017.08.083 Wibawa H. Saputera , Jason A. Scott , Donia Friedmann , Rose Amal
|
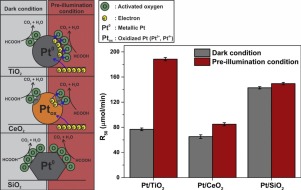
The role of key oxidative species in formic acid oxidation using neat and platinum-loaded TiO2, CeO2 and SiO2 was investigated. The catalytic reactions under three different illumination conditions; dark catalysis (i.e. no illumination), dark catalysis with UV light pre-treatment (denoted as pre-30) and photocatalysis (i.e. continuous UV illumination), were studied. The activities of neat TiO2, CeO2 and SiO2 were low under dark and pre-30 conditions while under illumination (photocatalysis) the semiconductor metal oxides, TiO2 and CeO2 showed much higher oxidation rates. For the Pt-loaded catalysts, the key active species were surface active oxygen (PtOads and O−ads) under the dark catalytic condition with and without light pre-treatment while under photocatalytic condition, photogenerated holes and electrons are believed to form hydroxyl radicals (OH) and superoxide radicals (
O2−), respectively, for CeO2, TiO2, Pt/TiO2 and Pt/CeO2. Unexpectedly, Pt/SiO2 showed the highest activity under the dark catalytic condition. The cuboctahedral shape of the Pt deposits on the SiO2 surface, which promote a greater number of sharp edges/corners at the interface, is believed to have been advantageous for dissociating and activating adsorbed oxygen species compared to the Pt deposits on TiO2 and CeO2. The Pt deposit shape is thought to be responsible for the observed high activity of Pt/SiO2 for the dark catalytic oxidation of formic acid.
中文翻译:

揭示在黑暗和明亮条件下载有Pt的金属氧化物产生的关键氧化物质
研究了主要氧化物种在纯净和负载铂的TiO 2,CeO 2和SiO 2氧化甲酸中的作用。在三种不同光照条件下的催化反应;研究了黑暗催化(即无照明),采用紫外线预处理的黑暗催化(表示为pre-30)和光催化(即连续UV照明)。纯的TiO 2,CeO 2和SiO 2的活性在黑暗和30℃以下条件下较低,而在光照(光催化)作用下,半导体金属氧化物TiO 2和CeO 2的活性较低。显示出更高的氧化速率。对于负载Pt的催化剂,关键的活性物质是在黑暗的催化条件下和没有进行光预处理的情况下的表面活性氧(PtO ads和O - ads),而在光催化条件下,光生空穴和电子被认为会形成羟基自由基(OH)和超氧自由基(
ö 2 - ),分别为的CeO 2,的TiO 2,铂/二氧化钛2和Pt /的CeO 2。出乎意料的是,Pt / SiO 2在黑暗催化条件下表现出最高的活性。Pt沉积在SiO 2上的立方八面体形状与在TiO 2和CeO 2上沉积的Pt相比,在界面处产生大量锐利边缘/角的表面被认为有利于离解和活化吸附的氧。据认为,Pt沉积物的形状是观察到的Pt / SiO 2对甲酸的暗催化氧化的高活性的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号