当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a New Zinc Binding Chemotype by Fragment Screening
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00606 Panagiotis K. Chrysanthopoulos 1 , Prashant Mujumdar 1 , Lucy A. Woods 1 , Olan Dolezal 2 , Bin Ren 2 , Thomas S. Peat 2 , Sally-Ann Poulsen 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-09-01 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00606 Panagiotis K. Chrysanthopoulos 1 , Prashant Mujumdar 1 , Lucy A. Woods 1 , Olan Dolezal 2 , Bin Ren 2 , Thomas S. Peat 2 , Sally-Ann Poulsen 1
Affiliation
|
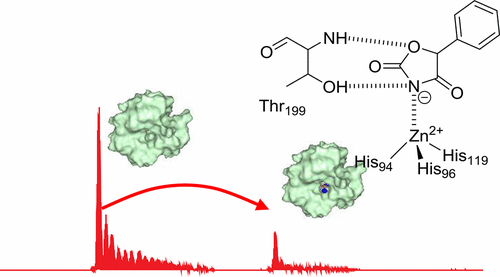
The discovery of a new zinc binding chemotype from screening a nonbiased fragment library is reported. Using the orthogonal fragment screening methods of native state mass spectrometry and surface plasmon resonance a 3-unsubstituted 2,4-oxazolidinedione fragment was found to have low micromolar binding affinity to the zinc metalloenzyme carbonic anhydrase II (CA II). This affinity approached that of fragment sized primary benzenesulfonamides, the classical zinc binding group found in most CA II inhibitors. Protein X-ray crystallography established that 3-unsubstituted 2,4-oxazolidinediones bound to CA II via an interaction of the acidic ring nitrogen with the CA II active site zinc, as well as two hydrogen bonds between the oxazolidinedione ring oxygen and the CA II protein backbone. Furthermore, 3-unsubstituted 2,4-oxazolidinediones appear to be a viable starting point for the development of an alternative class of CA inhibitor, wherein the medicinal chemistry pedigree of primary sulfonamides has dominated for several decades.
中文翻译:

通过片段筛选鉴定新的锌结合化学型
据报道,通过筛选无偏片段文库发现了一种新的锌结合化学型。使用天然质谱和表面等离子体共振的正交片段筛选方法,发现3-未取代的2,4-恶唑烷二酮片段对锌金属酶碳酸酐酶II(CA II)的微摩尔结合亲和力低。这种亲和力接近片段大小的伯苯磺酰胺的亲和力,这是大多数CA II抑制剂中发现的经典锌结合基团。蛋白质X射线晶体学确定,通过酸性环氮与CA II活性位点锌的相互作用以及恶唑烷二酮环氧与CA II之间的两个氢键,3-未取代的2,4-恶唑烷二酮与CA II结合。蛋白质骨架。此外,3-未取代的2
更新日期:2017-09-04
中文翻译:

通过片段筛选鉴定新的锌结合化学型
据报道,通过筛选无偏片段文库发现了一种新的锌结合化学型。使用天然质谱和表面等离子体共振的正交片段筛选方法,发现3-未取代的2,4-恶唑烷二酮片段对锌金属酶碳酸酐酶II(CA II)的微摩尔结合亲和力低。这种亲和力接近片段大小的伯苯磺酰胺的亲和力,这是大多数CA II抑制剂中发现的经典锌结合基团。蛋白质X射线晶体学确定,通过酸性环氮与CA II活性位点锌的相互作用以及恶唑烷二酮环氧与CA II之间的两个氢键,3-未取代的2,4-恶唑烷二酮与CA II结合。蛋白质骨架。此外,3-未取代的2











































 京公网安备 11010802027423号
京公网安备 11010802027423号