当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total synthesis of orientalol F via gold-catalyzed cycloisomerization of alkynediol
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7qo00654c Yueqing Gu 1, 2, 3, 4, 5 , Jun Huang 1, 2, 3, 4, 5 , Jianxian Gong 1, 2, 3, 4, 5 , Zhen Yang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7qo00654c Yueqing Gu 1, 2, 3, 4, 5 , Jun Huang 1, 2, 3, 4, 5 , Jianxian Gong 1, 2, 3, 4, 5 , Zhen Yang 1, 2, 3, 4, 5
Affiliation
|
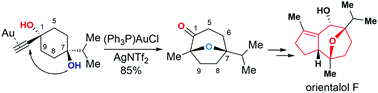
The total synthesis of orientalol F has been achieved starting from 1,4-dioxaspirodecan-8-one 11 in 13 steps. The key steps in this synthesis feature: (1) gold-catalyzed tandem cycloisomerization of alkynediol 10 for the formation of its seven-membered oxa-bridged bicyclic skeleton 9 of orientalol F, (2) visible-light-promoted organocatalytic aerobic oxidation of silyl enol ether 16 to enone 17, (3) Barbier-type butenylation for the diastereoselective synthesis of allylic alcohol 18 from enone 17, and (4) substrate-controlled Pd-catalyzed hydrogenation of 20 for the stereoselective installation of the C1 stereogenic center of 8.
中文翻译:

通过炔烃二醇的金催化环异构化全合成东方酚F
从1,4-二氧杂螺环烷-8-one 11开始,在13个步骤中完成了香酚F的全合成。该合成的关键步骤包括:(1)炔二醇10的金催化串联环异构化反应,以形成其七元酚F的七元氧桥双环骨架9;(2)可见光促进的甲硅烷基的有机催化需氧氧化烯醇醚16至烯酮17,(3)Barbier型丁烯化,用于从烯酮17非对映选择性合成烯丙醇18,以及(4)底物控制的Pd催化的20的氢化,以立体选择性地安装C1立体异构中心8。
更新日期:2017-09-04
中文翻译:

通过炔烃二醇的金催化环异构化全合成东方酚F
从1,4-二氧杂螺环烷-8-one 11开始,在13个步骤中完成了香酚F的全合成。该合成的关键步骤包括:(1)炔二醇10的金催化串联环异构化反应,以形成其七元酚F的七元氧桥双环骨架9;(2)可见光促进的甲硅烷基的有机催化需氧氧化烯醇醚16至烯酮17,(3)Barbier型丁烯化,用于从烯酮17非对映选择性合成烯丙醇18,以及(4)底物控制的Pd催化的20的氢化,以立体选择性地安装C1立体异构中心8。










































 京公网安备 11010802027423号
京公网安备 11010802027423号