当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An enantioselective conjugate addition reaction of 3-substituted benzothiophen-2-ones and 2-phthalimidoacrylates
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1039/c7qo00531h Shoulei Li 1, 2, 3, 4, 5 , Enge Zhang 1, 2, 3, 4, 5 , Junjun Feng 1, 2, 3, 4, 5 , Xin Li 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1039/c7qo00531h Shoulei Li 1, 2, 3, 4, 5 , Enge Zhang 1, 2, 3, 4, 5 , Junjun Feng 1, 2, 3, 4, 5 , Xin Li 1, 2, 3, 4, 5
Affiliation
|
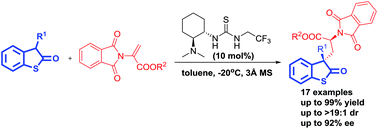
A highly enantioselective conjugate addition reaction of 3-substituted benzothiophen-2-ones to 2-phthalimidoacrylates has been developed using a bifunctional tertiary-amine thiourea catalyst. A number of chiral 3-substituted benzothiophen-2-one compounds were obtained with excellent yields (up to 99%) and very good stereoselectivities (up to >19 :
: 1 dr and up to 92% ee). The reaction was proved to be an efficient strategy for the synthesis of enantioenriched α-amino acid derivatives with 1,3-nonadjacent stereogenic centers.
1 dr and up to 92% ee). The reaction was proved to be an efficient strategy for the synthesis of enantioenriched α-amino acid derivatives with 1,3-nonadjacent stereogenic centers.
中文翻译:

3-取代的苯并噻吩-2-酮与2-邻苯二甲酰亚胺基丙烯酸酯的对映选择性共轭加成反应
已经使用双官能叔胺硫脲催化剂开发了3-取代的苯并噻吩-2-酮与2-邻苯二甲酰亚胺基丙烯酸酯的高度对映选择性共轭加成反应。具有优异的产率(高达99%)和非常好的立体选择性(高达> 19获得若干手性3-取代的苯并噻吩-2-酮化合物的 :
: 1个博士和高达92%ee)的。该反应被证明是合成具有1,3-不相邻立体异构中心的对映体富集的α-氨基酸衍生物的有效策略。
1个博士和高达92%ee)的。该反应被证明是合成具有1,3-不相邻立体异构中心的对映体富集的α-氨基酸衍生物的有效策略。
更新日期:2017-09-04
 :
: 1 dr and up to 92% ee). The reaction was proved to be an efficient strategy for the synthesis of enantioenriched α-amino acid derivatives with 1,3-nonadjacent stereogenic centers.
1 dr and up to 92% ee). The reaction was proved to be an efficient strategy for the synthesis of enantioenriched α-amino acid derivatives with 1,3-nonadjacent stereogenic centers.
中文翻译:

3-取代的苯并噻吩-2-酮与2-邻苯二甲酰亚胺基丙烯酸酯的对映选择性共轭加成反应
已经使用双官能叔胺硫脲催化剂开发了3-取代的苯并噻吩-2-酮与2-邻苯二甲酰亚胺基丙烯酸酯的高度对映选择性共轭加成反应。具有优异的产率(高达99%)和非常好的立体选择性(高达> 19获得若干手性3-取代的苯并噻吩-2-酮化合物的
 :
: 1个博士和高达92%ee)的。该反应被证明是合成具有1,3-不相邻立体异构中心的对映体富集的α-氨基酸衍生物的有效策略。
1个博士和高达92%ee)的。该反应被证明是合成具有1,3-不相邻立体异构中心的对映体富集的α-氨基酸衍生物的有效策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号