Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study.
The Lancet ( IF 168.9 ) Pub Date : 2017-10-01 , DOI: 10.1016/s1470-2045(17)30615-0 Uwe Platzbecker , Ulrich Germing , Katharina S Götze , Philipp Kiewe , Karin Mayer , Jörg Chromik , Markus Radsak , Thomas Wolff , Xiaosha Zhang , Abderrahmane Laadem , Matthew L Sherman , Kenneth M Attie , Aristoteles Giagounidis
The Lancet ( IF 168.9 ) Pub Date : 2017-10-01 , DOI: 10.1016/s1470-2045(17)30615-0 Uwe Platzbecker , Ulrich Germing , Katharina S Götze , Philipp Kiewe , Karin Mayer , Jörg Chromik , Markus Radsak , Thomas Wolff , Xiaosha Zhang , Abderrahmane Laadem , Matthew L Sherman , Kenneth M Attie , Aristoteles Giagounidis
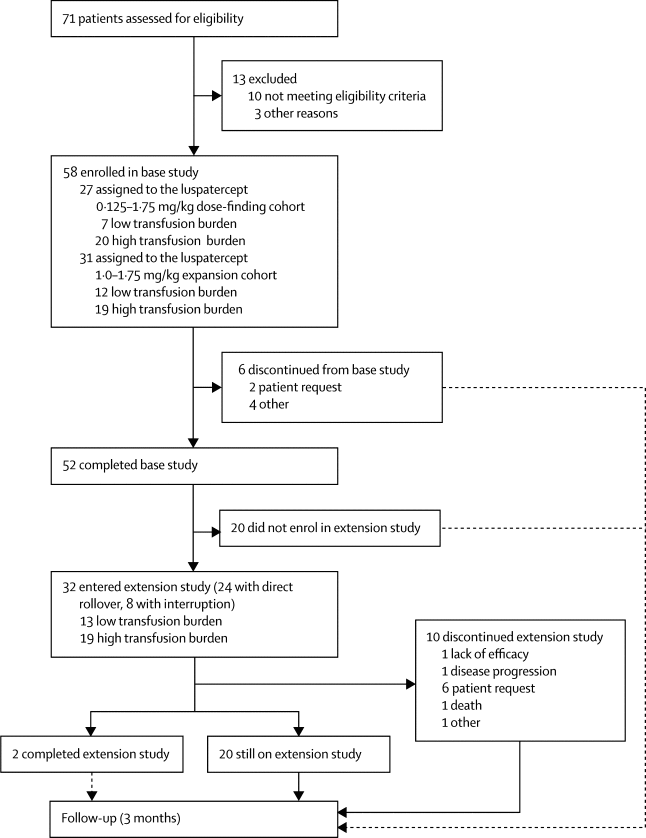
|
Myelodysplastic syndromes are characterised by ineffective erythropoiesis. Luspatercept (ACE-536) is a novel fusion protein that blocks transforming growth factor beta (TGF β) superfamily inhibitors of erythropoiesis, giving rise to a promising new investigative therapy. We aimed to assess the safety and efficacy of luspatercept in patients with anaemia due to lower-risk myelodysplastic syndromes.
中文翻译:

Luspatercept用于治疗低危骨髓增生异常综合症(PACE-MDS)贫血的患者:一项多中心,开放标签的2期剂量寻找研究,并进行了长期扩展研究。
骨髓增生异常综合症的特征是无效的红细胞生成。Luspatercept(ACE-536)是一种新型融合蛋白,可阻断促红细胞生成的转化生长因子β(TGFβ)超家族抑制剂,从而产生了有希望的新研究方法。我们旨在评估因低危骨髓增生异常综合征而导致的贫血患者接受luspatercept的安全性和有效性。
更新日期:2017-09-04
中文翻译:

Luspatercept用于治疗低危骨髓增生异常综合症(PACE-MDS)贫血的患者:一项多中心,开放标签的2期剂量寻找研究,并进行了长期扩展研究。
骨髓增生异常综合症的特征是无效的红细胞生成。Luspatercept(ACE-536)是一种新型融合蛋白,可阻断促红细胞生成的转化生长因子β(TGFβ)超家族抑制剂,从而产生了有希望的新研究方法。我们旨在评估因低危骨髓增生异常综合征而导致的贫血患者接受luspatercept的安全性和有效性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号