Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-08-18 , DOI: 10.1016/j.mcat.2017.08.006 Ravirala Ramu , Wondemagegn Hailemichael Wanna , Damodar Janmanchi , Yi-Fang Tsai , Chih-Cheng Liu , Chung-Yuan Mou , Steve S.-F. Yu
|
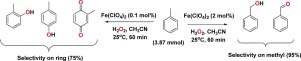
Iron(II) perchlorate in an H2O-H2O2-CH3CN mixture was used to efficiently carry out CH bond activations of benzene to form phenol and/or hydroquinone, and of toluene to form benzaldehyde, benzyl alcohol, o-cresol, p-cresol, and/or methyl-p-benzoquinone. The reactions were facilely tuned and controlled to selectively yield either a single or double oxygenation of benzene as well as a sp3 or sp2 C
H bond oxidation of toluene. On the basis of H/D kinetic isotope effect data, we determined the aromatic oxidation to mostly proceed by way of formation of an arene oxide or a σ-complex intermediate from high-valence iron species and to then undergo a 1,2-hydride shift, i.e., NIH-shift rearrangement.
中文翻译:

H 2 O 2 -H 2 O-CH 3 CN体系中Fe(ClO 4)2催化苯和甲苯选择性氧化的机理研究
铁(II),高氯酸中的H 2 O - H 2 ö 2 -CH 3 CN混合物用于至C有效地进行苯的H键激活以形成苯酚和/或氢醌,和甲苯以形成苯甲醛,苄醇,ö -甲酚,对甲酚和/或甲基对苯醌。可以轻松调节反应并进行控制,以选择性产生苯的单次或双次氧化以及sp 3或sp 2 C
甲苯的H键氧化。根据H / D动力学同位素效应数据,我们确定了芳族氧化反应主要是通过从高价铁物种形成氧化芳烃或σ-复杂中间体而进行的,然后进行1,2-氢化物移位,即NIH移位重排。











































 京公网安备 11010802027423号
京公网安备 11010802027423号