Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-08-16 , DOI: 10.1016/j.mcat.2017.07.004 Sérgio R. Tavares , Fernando Wypych , Alexandre A. Leitão
|
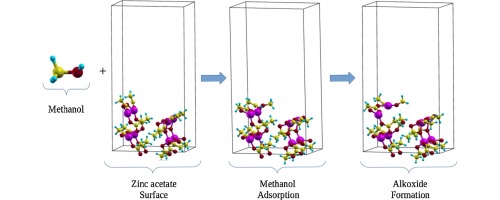
DFT calculations with periodic boundary conditions were performed in order to study the esterification/transesterification mechanisms involved in the biodiesel production. The simulation of the adsorptions of acetic acid, methanol and triacetin was carried out on zinc acetate as the catalyst. The adsorption energies of these processes could be obtained and PDOS of the zinc sites and the carboxylate group carbons of the acetic acid and the triacetin were explored for the evaluation of their acidity. The adsorption energies and the reaction barrier of the alkoxide formation showed that the triglyceride adsorption is very likely to occur first in transesterification processes. The barriers also denote that an alkoxide formation from the methanol is not favored and, consequently, a nucleophilic attack from the methanol molecule occurs.
中文翻译:

基于DFT的醋酸锌表面上乙酸,三醋精,甲醇和醇盐形成的吸附计算
为了研究生物柴油生产中涉及的酯化/酯交换机理,进行了具有周期性边界条件的DFT计算。以乙酸锌为催化剂对乙酸,甲醇和三醋精的吸附进行了模拟。可以获得这些过程的吸附能,并探索了乙酸和三醋精的锌位点的PDOS和羧酸根碳,以评估它们的酸度。醇盐形成的吸附能和反应势垒表明,甘油三酸酯的吸附极有可能在酯交换过程中首先发生。障碍还表示不希望由甲醇形成醇盐,因此,发生了由甲醇分子引起的亲核攻击。









































 京公网安备 11010802027423号
京公网安备 11010802027423号