Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Circuit Node that Integrates Convergent Input from Neuromodulatory and Social Behavior-Promoting Neurons to Control Aggression in Drosophila.
Neuron ( IF 14.7 ) Pub Date : 2017-Aug-30 , DOI: 10.1016/j.neuron.2017.08.017 Kiichi Watanabe 1 , Hui Chiu 1 , Barret D Pfeiffer 2 , Allan M Wong 2 , Eric D Hoopfer 3 , Gerald M Rubin 4 , David J Anderson 1
Neuron ( IF 14.7 ) Pub Date : 2017-Aug-30 , DOI: 10.1016/j.neuron.2017.08.017 Kiichi Watanabe 1 , Hui Chiu 1 , Barret D Pfeiffer 2 , Allan M Wong 2 , Eric D Hoopfer 3 , Gerald M Rubin 4 , David J Anderson 1
Affiliation
|
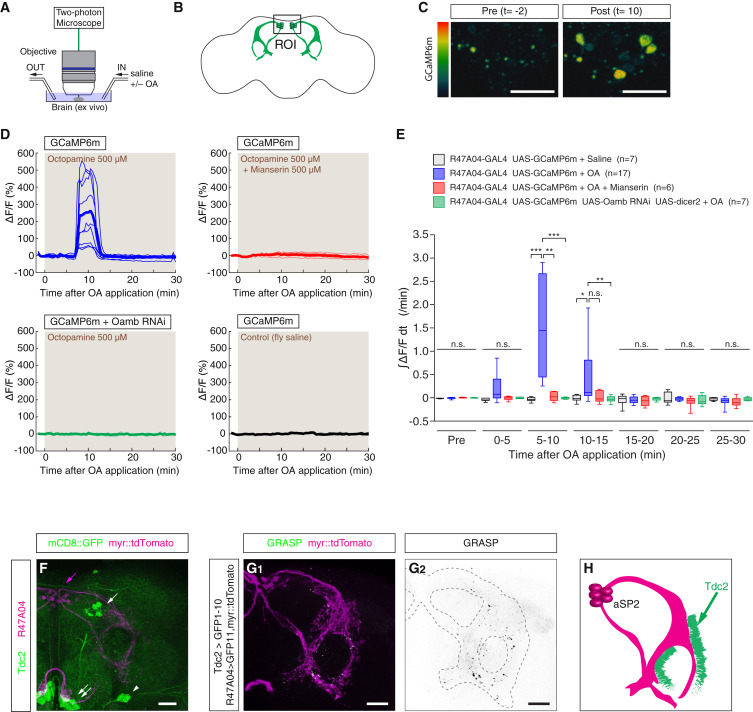
Diffuse neuromodulatory systems such as norepinephrine (NE) control brain-wide states such as arousal, but whether they control complex social behaviors more specifically is not clear. Octopamine (OA), the insect homolog of NE, is known to promote both arousal and aggression. We have performed a systematic, unbiased screen to identify OA receptor-expressing neurons (OARNs) that control aggression in Drosophila. Our results uncover a tiny population of male-specific aSP2 neurons that mediate a specific influence of OA on aggression, independent of any effect on arousal. Unexpectedly, these neurons receive convergent input from OA neurons and P1 neurons, a population of FruM+ neurons that promotes male courtship behavior. Behavioral epistasis experiments suggest that aSP2 neurons may constitute an integration node at which OAergic neuromodulation can bias the output of P1 neurons to favor aggression over inter-male courtship. These results have potential implications for thinking about the role of related neuromodulatory systems in mammals.
中文翻译:

整合神经调节和社会行为促进神经元的汇聚输入以控制果蝇攻击性的电路节点。
去甲肾上腺素(NE)等弥漫性神经调节系统控制着整个大脑的状态,例如觉醒,但它们是否更具体地控制复杂的社会行为尚不清楚。章鱼胺 (OA) 是 NE 的昆虫同系物,已知可促进兴奋和攻击性。我们进行了系统的、公正的筛选,以鉴定控制果蝇攻击性的表达 OA 受体的神经元 (OARN)。我们的结果揭示了一小群男性特有的 aSP2 神经元,它们介导 OA 对攻击性的特定影响,与对唤醒的任何影响无关。出乎意料的是,这些神经元接收来自 OA 神经元和 P1 神经元(一群促进雄性求爱行为的 FruM +神经元)的汇聚输入。行为上位实验表明,aSP2 神经元可能构成一个整合节点,OAergic 神经调节可以在该节点上偏向 P1 神经元的输出,使其有利于攻击性而不是雄性之间的求爱。这些结果对于思考哺乳动物中相关神经调节系统的作用具有潜在的意义。
更新日期:2017-08-31
中文翻译:

整合神经调节和社会行为促进神经元的汇聚输入以控制果蝇攻击性的电路节点。
去甲肾上腺素(NE)等弥漫性神经调节系统控制着整个大脑的状态,例如觉醒,但它们是否更具体地控制复杂的社会行为尚不清楚。章鱼胺 (OA) 是 NE 的昆虫同系物,已知可促进兴奋和攻击性。我们进行了系统的、公正的筛选,以鉴定控制果蝇攻击性的表达 OA 受体的神经元 (OARN)。我们的结果揭示了一小群男性特有的 aSP2 神经元,它们介导 OA 对攻击性的特定影响,与对唤醒的任何影响无关。出乎意料的是,这些神经元接收来自 OA 神经元和 P1 神经元(一群促进雄性求爱行为的 FruM +神经元)的汇聚输入。行为上位实验表明,aSP2 神经元可能构成一个整合节点,OAergic 神经调节可以在该节点上偏向 P1 神经元的输出,使其有利于攻击性而不是雄性之间的求爱。这些结果对于思考哺乳动物中相关神经调节系统的作用具有潜在的意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号