当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Assembly and Building Blocks: Beyond the Limits of the LEGO Brick Metaphor
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.biochem.7b00666 Yaakov Levy 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.biochem.7b00666 Yaakov Levy 1
Affiliation
|
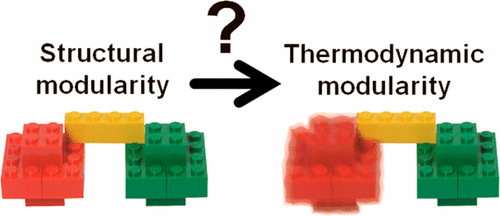
Proteins, like other biomolecules, have a modular and hierarchical structure. Various building blocks are used to construct proteins of high structural complexity and diverse functionality. In multidomain proteins, for example, domains are fused to each other in different combinations to achieve different functions. Although the LEGO brick metaphor is justified as a means of simplifying the complexity of three-dimensional protein structures, several fundamental properties (such as allostery or the induced-fit mechanism) make deviation from it necessary to respect the plasticity, softness, and cross-talk that are essential to protein function. In this work, we illustrate recently reported protein behavior in multidomain proteins that deviates from the LEGO brick analogy. While earlier studies showed that a protein domain is often unaffected by being fused to another domain or becomes more stable following the formation of a new interface between the tethered domains, destabilization due to tethering has been reported for several systems. We illustrate that tethering may sometimes result in a multidomain protein behaving as “less than the sum of its parts”. We survey these cases for which structure additivity does not guarantee thermodynamic additivity. Protein destabilization due to fusion to other domains may be linked in some cases to biological function and should be taken into account when designing large assemblies.
中文翻译:

蛋白质组装和构建基块:超越乐高积木隐喻的极限
蛋白质与其他生物分子一样,具有模块化和层次结构。各种构件被用来构建具有高结构复杂性和多样化功能的蛋白质。例如,在多结构域蛋白中,结构域以不同的组合彼此融合以实现不同的功能。尽管乐高积木的比喻被认为是简化三维蛋白质结构复杂性的一种手段,但是一些基本属性(例如变构或诱导拟合机制)使得必须偏离其范围,以尊重其可塑性,柔软性和交叉性。对蛋白质功能至关重要的对话。在这项工作中,我们举例说明了最近报道的与乐高积木类比不同的多域蛋白质中的蛋白质行为。尽管较早的研究表明,蛋白质结构域通常不会因与另一个结构域融合而受到影响,或者在拴系结构域之间形成新界面后变得更稳定,但已报道了几种系统由于拴系而造成的不稳定作用。我们举例说明,网络绑定有时可能会导致多域蛋白质的行为“小于其各个部分的总和”。我们调查了结构加性不能保证热力学加性的情况。在某些情况下,由于与其他结构域融合而引起的蛋白质不稳定可能与生物学功能有关,在设计大型装配时应予以考虑。我们举例说明,网络绑定有时可能会导致多域蛋白质的行为“小于其各个部分的总和”。我们调查了结构加性不能保证热力学加性的情况。在某些情况下,由于与其他结构域融合而引起的蛋白质不稳定可能与生物学功能有关,在设计大型装配时应予以考虑。我们举例说明,网络绑定有时可能会导致多域蛋白质的行为“小于其各个部分的总和”。我们调查了结构加性不能保证热力学加性的情况。在某些情况下,由于与其他结构域融合而引起的蛋白质不稳定可能与生物学功能有关,在设计大型装配时应予以考虑。
更新日期:2017-08-31
中文翻译:

蛋白质组装和构建基块:超越乐高积木隐喻的极限
蛋白质与其他生物分子一样,具有模块化和层次结构。各种构件被用来构建具有高结构复杂性和多样化功能的蛋白质。例如,在多结构域蛋白中,结构域以不同的组合彼此融合以实现不同的功能。尽管乐高积木的比喻被认为是简化三维蛋白质结构复杂性的一种手段,但是一些基本属性(例如变构或诱导拟合机制)使得必须偏离其范围,以尊重其可塑性,柔软性和交叉性。对蛋白质功能至关重要的对话。在这项工作中,我们举例说明了最近报道的与乐高积木类比不同的多域蛋白质中的蛋白质行为。尽管较早的研究表明,蛋白质结构域通常不会因与另一个结构域融合而受到影响,或者在拴系结构域之间形成新界面后变得更稳定,但已报道了几种系统由于拴系而造成的不稳定作用。我们举例说明,网络绑定有时可能会导致多域蛋白质的行为“小于其各个部分的总和”。我们调查了结构加性不能保证热力学加性的情况。在某些情况下,由于与其他结构域融合而引起的蛋白质不稳定可能与生物学功能有关,在设计大型装配时应予以考虑。我们举例说明,网络绑定有时可能会导致多域蛋白质的行为“小于其各个部分的总和”。我们调查了结构加性不能保证热力学加性的情况。在某些情况下,由于与其他结构域融合而引起的蛋白质不稳定可能与生物学功能有关,在设计大型装配时应予以考虑。我们举例说明,网络绑定有时可能会导致多域蛋白质的行为“小于其各个部分的总和”。我们调查了结构加性不能保证热力学加性的情况。在某些情况下,由于与其他结构域融合而引起的蛋白质不稳定可能与生物学功能有关,在设计大型装配时应予以考虑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号