当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Conformational Flexibility of the Acyltransferase from the Disorazole Polyketide Synthase Is Revealed by an X-ray Free-Electron Laser Using a Room-Temperature Sample Delivery Method for Serial Crystallography
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.biochem.7b00711 Irimpan I. Mathews 1 , Kim Allison 1 , Thomas Robbins , Artem Y. Lyubimov 1 , Monarin Uervirojnangkoorn , Axel T. Brunger , Chaitan Khosla , Hasan DeMirci 1 , Scott E. McPhillips 1 , Michael Hollenbeck 1 , Michael Soltis 1 , Aina E. Cohen 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.biochem.7b00711 Irimpan I. Mathews 1 , Kim Allison 1 , Thomas Robbins , Artem Y. Lyubimov 1 , Monarin Uervirojnangkoorn , Axel T. Brunger , Chaitan Khosla , Hasan DeMirci 1 , Scott E. McPhillips 1 , Michael Hollenbeck 1 , Michael Soltis 1 , Aina E. Cohen 1
Affiliation
|
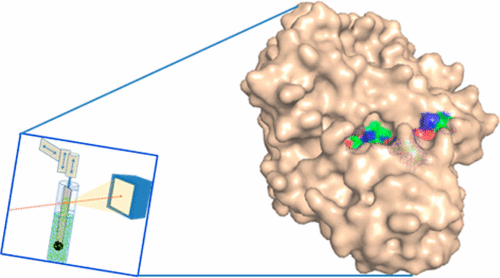
The crystal structure of the trans-acyltransferase (AT) from the disorazole polyketide synthase (PKS) was determined at room temperature to a resolution of 2.5 Å using a new method for the direct delivery of the sample into an X-ray free-electron laser. A novel sample extractor efficiently delivered limited quantities of microcrystals directly from the native crystallization solution into the X-ray beam at room temperature. The AT structure revealed important catalytic features of this core PKS enzyme, including the occurrence of conformational changes around the active site. The implications of these conformational changes for polyketide synthase reaction dynamics are discussed.
中文翻译:

X射线自由电子激光使用室温样品传递方法进行串行晶体学分析,揭示了二甲苯唑聚酮化合物合酶中酰基转移酶的构象柔性。
使用新方法将样品直接递送到X射线自由电子激光器中,在室温下将二异唑基聚酮化合物合酶(PKS)的反式酰基转移酶(AT)的晶体结构确定为2.5Å的分辨率。新型样品提取器可在室温下将有限数量的微晶直接从天然结晶溶液中有效地传递到X射线束中。AT结构揭示了该核心PKS酶的重要催化特征,包括在活性位点周围发生构象变化。讨论了这些构象变化对聚酮化合物合酶反应动力学的影响。
更新日期:2017-08-31
中文翻译:

X射线自由电子激光使用室温样品传递方法进行串行晶体学分析,揭示了二甲苯唑聚酮化合物合酶中酰基转移酶的构象柔性。
使用新方法将样品直接递送到X射线自由电子激光器中,在室温下将二异唑基聚酮化合物合酶(PKS)的反式酰基转移酶(AT)的晶体结构确定为2.5Å的分辨率。新型样品提取器可在室温下将有限数量的微晶直接从天然结晶溶液中有效地传递到X射线束中。AT结构揭示了该核心PKS酶的重要催化特征,包括在活性位点周围发生构象变化。讨论了这些构象变化对聚酮化合物合酶反应动力学的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号