当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of the Stability of the Histone H2A–H2B Dimer by H2A Tyr57 Phosphorylation
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.biochem.7b00504 Takuma Sueoka 1 , Gosuke Hayashi 1 , Akimitsu Okamoto 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.biochem.7b00504 Takuma Sueoka 1 , Gosuke Hayashi 1 , Akimitsu Okamoto 1, 2
Affiliation
|
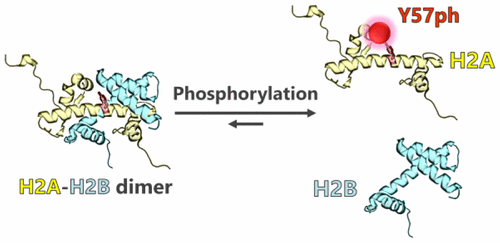
Histone H2A and H2B form a H2A–H2B heterodimer, which is a fundamental unit of nucleosome assembly and disassembly. Several posttranslational modifications change the interface between the H2A–H2B dimer and the H3–H4 tetramer and regulate nucleosome stability. However, posttranslational modifications associated with the interface between H2A and H2B have not been discussed. In this paper, it is shown that Tyr57 phosphorylation in H2A strongly influences H2A–H2B dimerization. Tyr57-phosphorylated H2A was chemically synthesized and utilized to reconstitute the H2A–H2B dimer and nucleosome as well as canonical H2A. Thermal shift assays showed that phosphorylation destabilized the dimer and facilitated dissociation of H2A and H2B from the nucleosome structure. The proximity between H2A Tyr57 and the H2B αC helix is assumed to lead the destabilization. The DNA accessibility of the nucleosome was estimated by using micrococcal nuclease. The phosphorylated nucleosome did not change DNA accessibility compared to that of the canonical nucleosome. It is demonstrated that phosphorylation at Tyr57 changes the H2A–H2B dimer interaction but does not interfere with histone–DNA interactions. This work on the destabilization of the H2A–H2B dimer by Tyr57 phosphorylation is a promising step in elucidating control mechanisms of dynamic behavior of H2A and H2B through posttranslational modifications.
中文翻译:

H2A Tyr57磷酸化对组蛋白H2A–H2B二聚体稳定性的调节
组蛋白H2A和H2B形成H2A–H2B异二聚体,这是核小体组装和拆卸的基本单元。几个翻译后修饰改变了H2A–H2B二聚体和H3–H4四聚体之间的界面,并调节了核小体的稳定性。但是,尚未讨论与H2A和H2B之间的界面相关的翻译后修饰。本文表明,H2A中的Tyr57磷酸化强烈影响H2A–H2B二聚化。Tyr57磷酸化的H2A是化学合成的,用于重构H2A–H2B二聚体和核小体以及经典H2A。热移分析显示磷酸化使二聚体不稳定,并促进H2A和H2B从核小体结构上解离。H2A Tyr57和H2BαC螺旋之间的接近被认为导致不稳定。通过使用微球菌核酸酶评估核小体的DNA可及性。磷酸化的核小体与标准核小体相比,不会改变DNA的可及性。结果表明,Tyr57的磷酸化改变了H2A–H2B二聚体的相互作用,但不干扰组蛋白与DNA的相互作用。通过Tyr57磷酸化对H2A–H2B二聚体进行去稳定化的这项工作是通过翻译后修饰阐明H2A和H2B动态行为控制机制的一个有希望的步骤。结果表明,Tyr57的磷酸化改变了H2A–H2B二聚体的相互作用,但不干扰组蛋白与DNA的相互作用。通过Tyr57磷酸化对H2A–H2B二聚体进行去稳定化的这项工作是通过翻译后修饰阐明H2A和H2B动态行为控制机制的一个有希望的步骤。结果表明,Tyr57的磷酸化改变了H2A–H2B二聚体的相互作用,但不干扰组蛋白与DNA的相互作用。通过Tyr57磷酸化对H2A–H2B二聚体进行去稳定化的这项工作是通过翻译后修饰阐明H2A和H2B动态行为控制机制的一个有希望的步骤。
更新日期:2017-08-31
中文翻译:

H2A Tyr57磷酸化对组蛋白H2A–H2B二聚体稳定性的调节
组蛋白H2A和H2B形成H2A–H2B异二聚体,这是核小体组装和拆卸的基本单元。几个翻译后修饰改变了H2A–H2B二聚体和H3–H4四聚体之间的界面,并调节了核小体的稳定性。但是,尚未讨论与H2A和H2B之间的界面相关的翻译后修饰。本文表明,H2A中的Tyr57磷酸化强烈影响H2A–H2B二聚化。Tyr57磷酸化的H2A是化学合成的,用于重构H2A–H2B二聚体和核小体以及经典H2A。热移分析显示磷酸化使二聚体不稳定,并促进H2A和H2B从核小体结构上解离。H2A Tyr57和H2BαC螺旋之间的接近被认为导致不稳定。通过使用微球菌核酸酶评估核小体的DNA可及性。磷酸化的核小体与标准核小体相比,不会改变DNA的可及性。结果表明,Tyr57的磷酸化改变了H2A–H2B二聚体的相互作用,但不干扰组蛋白与DNA的相互作用。通过Tyr57磷酸化对H2A–H2B二聚体进行去稳定化的这项工作是通过翻译后修饰阐明H2A和H2B动态行为控制机制的一个有希望的步骤。结果表明,Tyr57的磷酸化改变了H2A–H2B二聚体的相互作用,但不干扰组蛋白与DNA的相互作用。通过Tyr57磷酸化对H2A–H2B二聚体进行去稳定化的这项工作是通过翻译后修饰阐明H2A和H2B动态行为控制机制的一个有希望的步骤。结果表明,Tyr57的磷酸化改变了H2A–H2B二聚体的相互作用,但不干扰组蛋白与DNA的相互作用。通过Tyr57磷酸化对H2A–H2B二聚体进行去稳定化的这项工作是通过翻译后修饰阐明H2A和H2B动态行为控制机制的一个有希望的步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号