当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating Substrate Promiscuity within the FabI Enzyme Family
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acschembio.7b00400 Gabriel S. Freund 1, 2 , Terrence E. O’Brien 1, 3 , Logan Vinson 1 , Dylan Alexander Carlin 1, 4 , Andrew Yao 1 , Wai Shun Mak 1, 3 , Ilias Tagkopoulos 1, 5 , Marc T. Facciotti 1, 6 , Dean J. Tantillo 3 , Justin B. Siegel 1, 3, 7
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acschembio.7b00400 Gabriel S. Freund 1, 2 , Terrence E. O’Brien 1, 3 , Logan Vinson 1 , Dylan Alexander Carlin 1, 4 , Andrew Yao 1 , Wai Shun Mak 1, 3 , Ilias Tagkopoulos 1, 5 , Marc T. Facciotti 1, 6 , Dean J. Tantillo 3 , Justin B. Siegel 1, 3, 7
Affiliation
|
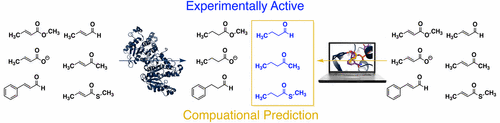
The rapidly growing appreciation of enzymes’ catalytic and substrate promiscuity may lead to their expanded use in the fields of chemical synthesis and industrial biotechnology. Here, we explore the substrate promiscuity of enoyl-acyl carrier protein reductases (commonly known as FabI) and how that promiscuity is a function of inherent reactivity and the geometric demands of the enzyme’s active site. We demonstrate that these enzymes catalyze the reduction of a wide range of substrates, particularly α,β-unsaturated aldehydes. In addition, we demonstrate that a combination of quantum mechanical hydride affinity calculations and molecular docking can be used to rapidly categorize compounds that FabI can use as substrates. The results here provide new insight into the determinants of catalysis for FabI and set the stage for the development of a new assay for drug discovery, organic synthesis, and novel biocatalysts.
中文翻译:

阐明FabI酶家族中的底物混杂
对酶的催化和底物混杂度的迅速增长的认识可能导致它们在化学合成和工业生物技术领域中的广泛使用。在这里,我们探讨了烯酰基-酰基载体蛋白还原酶(俗称FabI)的底物混杂性,以及该混杂性是固有反应性和酶活性位点的几何要求的函数的原因。我们证明这些酶催化多种底物,特别是α,β-不饱和醛的还原。此外,我们证明了量子机械氢化物亲和力计算和分子对接的组合可用于快速分类FabI可用作底物的化合物。
更新日期:2017-08-31
中文翻译:

阐明FabI酶家族中的底物混杂
对酶的催化和底物混杂度的迅速增长的认识可能导致它们在化学合成和工业生物技术领域中的广泛使用。在这里,我们探讨了烯酰基-酰基载体蛋白还原酶(俗称FabI)的底物混杂性,以及该混杂性是固有反应性和酶活性位点的几何要求的函数的原因。我们证明这些酶催化多种底物,特别是α,β-不饱和醛的还原。此外,我们证明了量子机械氢化物亲和力计算和分子对接的组合可用于快速分类FabI可用作底物的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号