Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2017-08-30 , DOI: 10.1016/j.apcata.2017.08.038 Tae Wan Kim , Jinho Oh , Young-Woong Suh
|
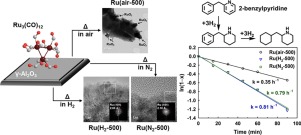
Although Ru3(CO)12 becomes a popular precursor for supported Ru catalysts nowadays, the activities of the catalysts prepared by thermolysis of the supported Ru3(CO)12 under different atmospheres have been rarely compared. We herein report the preparation of alumina-supported Ru samples by thermal activation of Ru3(CO)12 in air, H2 or N2, followed by activity test in the hydrogenation of 2-benzylpyridine (BPy). When the supported Ru3(CO)12 was activated in air, RuO2 particles of 12–15 nm diameters were produced by complete oxidation of carbonyl groups. In contrast, thermal activation in H2 and N2 induced the formation of highly dispersed Ru0 particles of 1.4–2.3 nm diameters. In such activations methane was produced, suggesting that direct hydrogenation of CO coordinated to the Ru surface complex occurred in H2 while the coordinated CO reacted with ruthenium hydride species in N2. In the activity test for BPy hydrogenation, the samples prepared in H2 and N2 showed superior H2 storage efficiencies and higher rate constants compared to those prepared in air (reduced before the reaction). Additionally, the former samples were examined to be relatively stable even though exposed to ambient air for 7 days. Therefore, H2 and N2 gases are recommended for thermal activation of alumina-supported Ru3(CO)12.
中文翻译:

在氧化铝负载的Ru催化剂上对2-苄基吡啶进行加氢:Ru 3(CO)12作为Ru前体的用途
尽管如今,Ru 3(CO)12成为负载型Ru催化剂的流行前体,但很少有人比较了在不同气氛下通过热解负载型Ru 3(CO)12制备的催化剂的活性。我们在此报告通过在空气,H 2或N 2中热活化Ru 3(CO)12,然后在2-苄基吡啶(BPy)氢化中进行活性测试,制备氧化铝负载的Ru样品。当负载的Ru 3(CO)12在空气中被激活时,RuO 2羰基完全氧化产生直径为12-15 nm的颗粒。相反,在H 2和N 2中的热活化导致形成高度分散的直径为1.4-2.3 nm的Ru 0粒子。在这种活化中,产生了甲烷,这表明配位至Ru表面配合物的CO的直接氢化发生在H 2中,而配位的CO与N 2中的氢化钌物质发生了反应。在BPy氢化活性测试中,在H 2和N 2中制备的样品显示出优异的H 2与在空气中制备的相比(在反应前降低),具有更高的储存效率和更高的速率常数。另外,即使暴露于环境空气中7天,仍检查了前者的样品是否相对稳定。因此,建议使用H 2和N 2气体对氧化铝负载的Ru 3(CO)12进行热活化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号