当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc-Catalyzed Dehydrogenative Silylation of Indoles
Organometallics ( IF 2.5 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.organomet.7b00382 Kyohei Yonekura 1 , Yoshihiko Iketani 1 , Masaru Sekine 1 , Tomohiro Tani 1 , Fumiya Matsui 1 , Daiki Kamakura 1 , Teruhisa Tsuchimoto 1
Organometallics ( IF 2.5 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.organomet.7b00382 Kyohei Yonekura 1 , Yoshihiko Iketani 1 , Masaru Sekine 1 , Tomohiro Tani 1 , Fumiya Matsui 1 , Daiki Kamakura 1 , Teruhisa Tsuchimoto 1
Affiliation
|
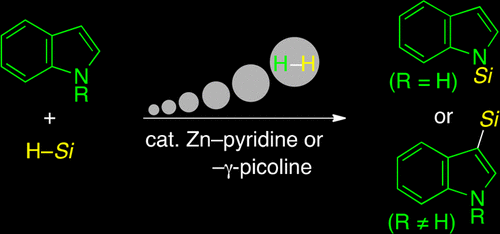
A unique Lewis acid/base system consisting of zinc triflate and pyridine was found to act as an effective catalyst for making an N(indolyl)–Si bond in a dehydrogenative fashion. Execution in a nitrile medium brings out the best performance of the Zn–pyridine system, which enables participation of flexible pieces of indoles and hydrosilanes, thereby giving diverse N-silylindoles in high to excellent yields. The Zn–pyridine system in the nitrile solvent is also applicable to the corresponding C-silylation in the case that the nitrogen atom of indoles has a substituent. Pyrrole, carbazole, arylamine, and thiophene substrates other than indoles undergo the dehydrogenative N- and/or C-silylation as well. Mechanistic studies showed that the role of the zinc Lewis acid is to activate the hydrosilane. The rate-determining step of the present reaction was found to be involved in the stage of the indolyl–H bond cleavage, on the basis of kinetic isotope effect experiments. Kinetic studies indicated that the indole-based dehydrogenative N-silylation is first-order in indole, second-order in each of hydrosilane and zinc triflate, and positive and negative fractional orders in pyridine.
中文翻译:

锌催化的吲哚脱氢硅烷化
发现由三氟甲磺酸锌和吡啶组成的独特的路易斯酸/碱体系可以有效地以脱氢方式形成N(吲哚基)-Si键。在腈介质中执行可使Zn-吡啶体系发挥出最佳性能,从而使吲哚和氢硅烷的柔性碎片得以参与,从而产生多样化的氮。-silylindoles高至优异的产量。在吲哚的氮原子具有取代基的情况下,腈溶剂中的Zn-吡啶体系也适用于相应的C-甲硅烷基化。除了吲哚以外,吡咯,咔唑,芳基胺和噻吩的底物也会进行脱氢的N-和/或C-甲硅烷基化。机理研究表明,路易斯酸锌的作用是活化氢硅烷。在动力学同位素效应实验的基础上,本反应的速率确定步骤被发现参与吲哚基-H键裂解的阶段。动力学研究表明,基于吲哚的脱氢N-甲硅烷基化在吲哚中为一阶,在氢硅烷和三氟甲磺酸锌中分别为二阶,在吡啶中为正分数级和负分数级。
更新日期:2017-08-31
中文翻译:

锌催化的吲哚脱氢硅烷化
发现由三氟甲磺酸锌和吡啶组成的独特的路易斯酸/碱体系可以有效地以脱氢方式形成N(吲哚基)-Si键。在腈介质中执行可使Zn-吡啶体系发挥出最佳性能,从而使吲哚和氢硅烷的柔性碎片得以参与,从而产生多样化的氮。-silylindoles高至优异的产量。在吲哚的氮原子具有取代基的情况下,腈溶剂中的Zn-吡啶体系也适用于相应的C-甲硅烷基化。除了吲哚以外,吡咯,咔唑,芳基胺和噻吩的底物也会进行脱氢的N-和/或C-甲硅烷基化。机理研究表明,路易斯酸锌的作用是活化氢硅烷。在动力学同位素效应实验的基础上,本反应的速率确定步骤被发现参与吲哚基-H键裂解的阶段。动力学研究表明,基于吲哚的脱氢N-甲硅烷基化在吲哚中为一阶,在氢硅烷和三氟甲磺酸锌中分别为二阶,在吡啶中为正分数级和负分数级。











































 京公网安备 11010802027423号
京公网安备 11010802027423号