当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mannobiose Binding Induces Changes in Hydrogen Bonding and Protonation States of Acidic Residues in Concanavalin A As Revealed by Neutron Crystallography
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.biochem.7b00654 Oksana O. Gerlits 1 , Leighton Coates 2 , Robert J. Woods 3 , Andrey Kovalevsky 2
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.biochem.7b00654 Oksana O. Gerlits 1 , Leighton Coates 2 , Robert J. Woods 3 , Andrey Kovalevsky 2
Affiliation
|
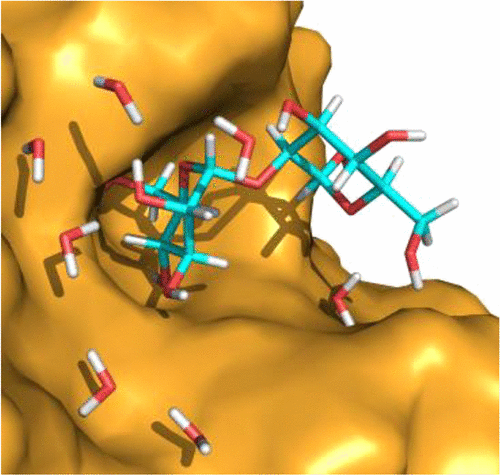
Plant lectins are carbohydrate-binding proteins with various biomedical applications. Concanavalin A (Con A) holds promise in treating cancerous tumors. To better understand the Con A carbohydrate binding specificity, we obtained a room-temperature neutron structure of this legume lectin in complex with a disaccharide Manα1–2Man, mannobiose. The neutron structure afforded direct visualization of the hydrogen bonding between the protein and ligand, showing that the ligand is able to alter both protonation states and interactions for residues located close to and distant from the binding site. An unprecedented low-barrier hydrogen bond was observed forming between the carboxylic side chains of Asp28 and Glu8, with the D atom positioned equidistant from the oxygen atoms having an O···D···O angle of 101.5°.
中文翻译:

甘露二糖结合诱导伴刀豆球蛋白A中酸性残基的氢键和质子化状态的变化,如中子晶体学所揭示
植物凝集素是具有各种生物医学应用的碳水化合物结合蛋白。伴刀豆球蛋白A(Con A)在治疗癌性肿瘤方面有希望。为了更好地了解Con A碳水化合物的结合特异性,我们获得了这种豆科植物凝集素与二糖Manα1-2Man(甘露二糖)的复合物的室温中子结构。中子结构提供了蛋白质和配体之间氢键的直接可视化,表明配体能够改变质子化状态以及位于靠近和远离结合位点的残基的相互作用。观察到在Asp28和Glu8的羧基侧链之间形成了前所未有的低势垒氢键,其中D原子与氧原子等距分布的O···D··O角为101.5°。
更新日期:2017-08-30
中文翻译:

甘露二糖结合诱导伴刀豆球蛋白A中酸性残基的氢键和质子化状态的变化,如中子晶体学所揭示
植物凝集素是具有各种生物医学应用的碳水化合物结合蛋白。伴刀豆球蛋白A(Con A)在治疗癌性肿瘤方面有希望。为了更好地了解Con A碳水化合物的结合特异性,我们获得了这种豆科植物凝集素与二糖Manα1-2Man(甘露二糖)的复合物的室温中子结构。中子结构提供了蛋白质和配体之间氢键的直接可视化,表明配体能够改变质子化状态以及位于靠近和远离结合位点的残基的相互作用。观察到在Asp28和Glu8的羧基侧链之间形成了前所未有的低势垒氢键,其中D原子与氧原子等距分布的O···D··O角为101.5°。











































 京公网安备 11010802027423号
京公网安备 11010802027423号