当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregation and Fibril Structure of AβM01–42 and Aβ1–42
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.biochem.7b00729 Robert Silvers 1 , Michael T. Colvin 1 , Kendra K. Frederick 2 , Angela C. Jacavone 1 , Susan Lindquist 2 , Sara Linse 3 , Robert G. Griffin 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.biochem.7b00729 Robert Silvers 1 , Michael T. Colvin 1 , Kendra K. Frederick 2 , Angela C. Jacavone 1 , Susan Lindquist 2 , Sara Linse 3 , Robert G. Griffin 1
Affiliation
|
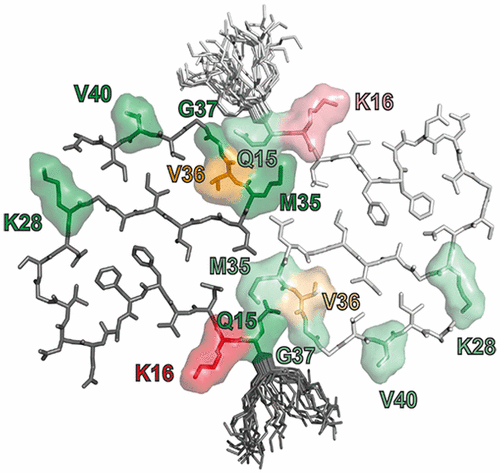
A mechanistic understanding of Aβ aggregation and high-resolution structures of Aβ fibrils and oligomers are vital to elucidating relevant details of neurodegeneration in Alzheimer’s disease, which will facilitate the rational design of diagnostic and therapeutic protocols. The most detailed and reproducible insights into structure and kinetics have been achieved using Aβ peptides produced by recombinant expression, which results in an additional methionine at the N-terminus. While the length of the C-terminus is well established to have a profound impact on the peptide’s aggregation propensity, structure, and neurotoxicity, the impact of the N-terminal methionine on the aggregation pathways and structure is unclear. For this reason, we have developed a protocol to produce recombinant Aβ1–42, sans the N-terminal methionine, using an N-terminal small ubiquitin-like modifier–Aβ1–42 fusion protein in reasonable yield, with which we compared aggregation kinetics with AβM01–42 containing the additional methionine residue. The data revealed that Aβ1–42 and AβM01–42 aggregate with similar rates and by the same mechanism, in which the generation of new aggregates is dominated by secondary nucleation of monomers on the surface of fibrils. We also recorded magic angle spinning nuclear magnetic resonance spectra that demonstrated that excellent spectral resolution is maintained with both AβM01–42 and Aβ1–42 and that the chemical shifts are virtually identical in dipolar recoupling experiments that provide information about rigid residues. Collectively, these results indicate that the structure of the fibril core is unaffected by N-terminal methionine. This is consistent with the recent structures of AβM01–42 in which M0 is located at the terminus of a disordered 14-amino acid N-terminal tail.
中文翻译:

AβM01–42和Aβ1–42的聚集和原纤维结构
对Aβ聚集以及Aβ原纤维和寡聚体的高分辨率结构的机械理解对于阐明阿尔茨海默氏病中神经退行性变的相关细节至关重要,这将有助于合理设计诊断和治疗方案。使用重组表达产生的Aβ肽已经获得了对结构和动力学的最详细和可再现的见解,这在N端产生了一个额外的蛋氨酸。虽然C端的长度已确定对肽的聚集倾向,结构和神经毒性有深远影响,但N末端甲硫氨酸对聚集途径和结构的影响尚不清楚。因此,我们已经制定了生产重组Aβ1–42的方案,SAN的N-末端甲硫氨酸,使用N末端小泛素样变性剂的Aβ 1-42在合理产率的融合蛋白,与我们比较聚集动力学与Aβ M01-42含有额外的甲硫氨酸残基。数据显示,Aβ1–42和AβM01–42以相似的速率和相同的机理聚集,其中新聚集体的产生主要由原纤维表面上的单体二次成核形成。我们还记录了魔角旋转核磁共振光谱,证明了AβM01–42和Aβ1–42均保持了出色的光谱分辨率并且在偶极耦合实验中化学位移实际上是相同的,该实验提供了有关刚性残基的信息。总的来说,这些结果表明原纤维核心的结构不受N-末端甲硫氨酸的影响。这与AβM01-42的最新结构是一致的,其中M0位于无序的14个氨基酸的N末端尾部的末端。
更新日期:2017-08-30
中文翻译:

AβM01–42和Aβ1–42的聚集和原纤维结构
对Aβ聚集以及Aβ原纤维和寡聚体的高分辨率结构的机械理解对于阐明阿尔茨海默氏病中神经退行性变的相关细节至关重要,这将有助于合理设计诊断和治疗方案。使用重组表达产生的Aβ肽已经获得了对结构和动力学的最详细和可再现的见解,这在N端产生了一个额外的蛋氨酸。虽然C端的长度已确定对肽的聚集倾向,结构和神经毒性有深远影响,但N末端甲硫氨酸对聚集途径和结构的影响尚不清楚。因此,我们已经制定了生产重组Aβ1–42的方案,SAN的N-末端甲硫氨酸,使用N末端小泛素样变性剂的Aβ 1-42在合理产率的融合蛋白,与我们比较聚集动力学与Aβ M01-42含有额外的甲硫氨酸残基。数据显示,Aβ1–42和AβM01–42以相似的速率和相同的机理聚集,其中新聚集体的产生主要由原纤维表面上的单体二次成核形成。我们还记录了魔角旋转核磁共振光谱,证明了AβM01–42和Aβ1–42均保持了出色的光谱分辨率并且在偶极耦合实验中化学位移实际上是相同的,该实验提供了有关刚性残基的信息。总的来说,这些结果表明原纤维核心的结构不受N-末端甲硫氨酸的影响。这与AβM01-42的最新结构是一致的,其中M0位于无序的14个氨基酸的N末端尾部的末端。











































 京公网安备 11010802027423号
京公网安备 11010802027423号