PLOS Medicine ( IF 10.5 ) Pub Date : 2017-08-29 , DOI: 10.1371/journal.pmed.1002379 Nicholas M Douglas 1, 2 , Jeanne Rini Poespoprodjo 3, 4 , Dewi Patriani 4 , Michael J Malloy 5, 6 , Enny Kenangalem 3, 7 , Paulus Sugiarto 8 , Julie A Simpson 5 , Yati Soenarto 4 , Nicholas M Anstey 1 , Ric N Price 1, 9
|
Background
Primaquine is the only licensed drug for eradicating Plasmodium vivax hypnozoites and, therefore, preventing relapses of vivax malaria. It is a vital component of global malaria elimination efforts. Primaquine is efficacious when supervised in clinical trials, but its effectiveness in real-world settings is unknown. We aimed to determine whether unsupervised primaquine was effective for preventing re-presentation to hospital with vivax malaria in southern Papua, Indonesia.
Methods and findings
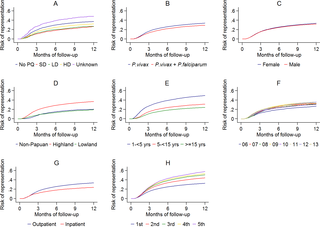
Routinely-collected hospital surveillance data were used to undertake a pragmatic comparison of the risk of re-presentation to hospital with vivax malaria in patients prescribed dihydroartemisinin-piperaquine (DHP) combined with primaquine versus those patients prescribed DHP alone. The omission of primaquine was predominantly due to 3 stock outages. Individual clinical, pharmacy, and laboratory data were merged using individual hospital identification numbers and the date of presentation to hospital. Between April 2004 and December 2013, there were 86,797 documented episodes of vivax malaria, of which 62,492 (72.0%) were included in the analysis. The risk of re-presentation with vivax malaria within 1 year was 33.8% (95% confidence Interval [CI] 33.1%–34.5%) after initial monoinfection with P. vivax and 29.2% (95% CI 28.1%–30.4%) after mixed-species infection. The risk of re-presentation with P. vivax malaria was higher in children 1 to <5 years of age (49.6% [95% CI 48.4%–50.9%]) compared to patients 15 years of age or older (24.2% [95% CI 23.4–24.9%]); Adjusted Hazard Ratio (AHR) = 2.23 (95% CI 2.15–2.31), p < 0.001. Overall, the risk of re-presentation was 37.2% (95% CI 35.6%–38.8%) in patients who were prescribed no primaquine compared to 31.6% (95% CI 30.9%–32.3%) in those prescribed either a low (≥1.5 mg/kg and <5 mg/kg) or high (≥5 mg/kg) dose of primaquine (AHR = 0.90 [95% CI 0.86–0.95, p < 0.001]). Limiting the comparison to high dose versus no primaquine in the period during and 12 months before and after a large stock outage resulted in minimal change in the estimated clinical effectiveness of primaquine (AHR 0.91, 95% CI 0.85–0.97, p = 0.003). Our pragmatic study avoided the clinical influences associated with prospective study involvement but was subject to attrition bias caused by passive follow-up.
Conclusions
Unsupervised primaquine for vivax malaria, prescribed according to the current World Health Organization guidelines, was associated with a minimal reduction in the risk of clinical recurrence within 1 year in Papua, Indonesia. New strategies for the effective radical cure of vivax malaria are needed in resource-poor settings.
中文翻译:

无监督伯氨喹治疗巴布亚南部间日疟原虫疟疾复发:一项以医院为基础的队列研究
背景
伯氨喹是唯一用于根除间日疟原虫休眠体并因此预防间日疟疾复发的许可药物。它是全球消除疟疾工作的重要组成部分。伯氨喹在临床试验的监督下是有效的,但其在现实环境中的有效性尚不清楚。我们的目的是确定在印度尼西亚巴布亚南部,无人监督的伯氨喹是否能有效防止因间日疟疾再次入院。
方法和结果
常规收集的医院监测数据用于对处方双氢青蒿素哌喹 (DHP) 联合伯氨喹的患者与单独处方 DHP 的患者因间日疟疾再次入院的风险进行务实比较。伯氨喹的遗漏主要是由于 3 次库存中断。个体临床、药学和实验室数据使用个体医院识别号和就诊日期合并。2004 年 4 月至 2013 年 12 月期间,有 86,797 起记录在案的间日疟疾发作,其中 62,492 起 (72.0%) 被纳入分析。初次单一感染疟原虫后,1 年内再次出现间日疟疾的风险为 33.8%(95% 置信区间 [CI] 33.1%–34.5% )。vivax和 29.2% (95% CI 28.1%–30.4%) 在混合物种感染后。用P重新呈现的风险。与 15 岁或以上的患者(24.2% [95% CI 23.4-24.9%])相比,1 至 5 岁以下儿童的间日疟疾发病率更高(49.6% [95% CI 48.4%–50.9%]);调整后的风险比 (AHR) = 2.23 (95% CI 2.15–2.31),p < 0.001。总体而言,未服用伯氨喹的患者再次出现的风险为 37.2%(95% CI 35.6%–38.8%),而服用低(≥ 1.5 mg/kg 和 <5 mg/kg) 或高 (≥5 mg/kg) 剂量的伯氨喹 (AHR = 0.90 [95% CI 0.86–0.95, p< 0.001])。在大量库存中断期间和之前和之后的 12 个月内将高剂量与无伯氨喹的比较限制在伯氨喹的估计临床有效性的估计变化很小(AHR 0.91,95% CI 0.85–0.97,p = 0.003)。我们的实用性研究避免了与前瞻性研究相关的临床影响,但受到被动随访引起的损耗偏倚的影响。
结论
在印度尼西亚巴布亚,根据现行世界卫生组织指南开具的无监督间日疟疾伯氨喹与 1 年内临床复发风险的最小降低有关。在资源匮乏的环境中,需要有效根治间日疟疾的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号