当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoswitchable Arylazopyrazole-Based Ruthenium(II) Arene Complexes
Organometallics ( IF 2.5 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.organomet.7b00493 Kesete Ghebreyessus 1 , Stefan M. Cooper 1
Organometallics ( IF 2.5 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.organomet.7b00493 Kesete Ghebreyessus 1 , Stefan M. Cooper 1
Affiliation
|
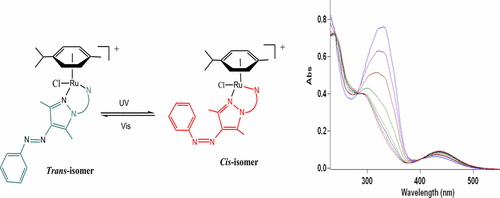
A new family of donor-functionalized photoswitchable arylazopyrazole-based ligands (3–5) was synthesized and characterized. The new ligands have been employed to prepare a series of novel photoswitchable half-sandwich ruthenium(II) cymene complexes of the type [(η6-p-cymene)Ru(L)Cl]+ (L = 1-(2-methylenepyridyl)-4-(phenyldiazenyl)-3,5-dimethyl-1H-pyrazole (6a), 1-(2-methylenepyridyl)-4-((4-bromophenyl)diazenyl)-3,5-dimethyl-1H-pyrazole (6b), 1-(2-benzothiazolyl)-3,5-dimethyl-4-arylazopyrazole (7)). All of the complexes have been fully characterized by 1H NMR, 13C NMR, and UV–vis spectroscopy and elemental analyses. In addition, the structure of complex 6a was determined by X-ray crystallography. The UV–vis spectroscopic studies show that both the ligands and metal complexes exhibit excellent trans to cis photoisomerization of the arylazopyrazole moiety upon irradiation with 365 nm UV light. The cis isomer of the compounds can be switched back nearly quantitatively to the more stable trans form with 530 nm irradiation. Coordination of the metal ion has no significant influence on the photoswitching properties of the ligands. DFT and TD-DFT calculations were performed for geometry optimization of the ligands and to complement the experimental findings of the electronic transitions and absorption bands observed. The data obtained from these studies were in good agreement with the experimental results. These excellent photoswitchable properties make the new cationic Ru(II) azo compounds described in these studies interesting candidates for their potential application as photoswitchable systems in catalytic and medicinal chemistry.
中文翻译:

光开关芳基吡唑基钌(II)芳烃配合物
供体官能化的光可基于arylazopyrazole配体(的一类新的3 - 5)的合成和表征。新的配位体已被用于制备一系列所述类型的新的光可半夹心钌(II)配合物伞花烃的[(η 6 - p -cymene)的Ru(L)CL] +(L = 1-(2- methylenepyridyl基)-4-(苯基二)-3,5-二甲基- 1 H ^ -吡唑(图6a),1-(2- methylenepyridyl)-4 - ((4-溴苯基)二氮烯基)-3,5-二甲基- 1 H ^ -吡唑(6b),1-(2-苯并噻唑基)-3,5-二甲基-4-芳基偶氮吡唑(7))。所有复合物均具有以下特征:1H NMR,13 C NMR和紫外可见光谱及元素分析。另外,复合物6a的结构通过X射线晶体学测定。紫外可见光谱研究表明,配体和金属配合物在365 nm紫外线照射下均表现出优异的芳基偶氮吡唑部分反式至顺式光异构化。化合物的顺式异构体可以在530 nm辐射下几乎定量地转换回更稳定的转化形式。金属离子的配位对配体的光开关性能没有显着影响。进行DFT和TD-DFT计算以优化配体的几何形状,并补充观察到的电子跃迁和吸收带的实验结果。从这些研究中获得的数据与实验结果非常吻合。
更新日期:2017-08-29
中文翻译:

光开关芳基吡唑基钌(II)芳烃配合物
供体官能化的光可基于arylazopyrazole配体(的一类新的3 - 5)的合成和表征。新的配位体已被用于制备一系列所述类型的新的光可半夹心钌(II)配合物伞花烃的[(η 6 - p -cymene)的Ru(L)CL] +(L = 1-(2- methylenepyridyl基)-4-(苯基二)-3,5-二甲基- 1 H ^ -吡唑(图6a),1-(2- methylenepyridyl)-4 - ((4-溴苯基)二氮烯基)-3,5-二甲基- 1 H ^ -吡唑(6b),1-(2-苯并噻唑基)-3,5-二甲基-4-芳基偶氮吡唑(7))。所有复合物均具有以下特征:1H NMR,13 C NMR和紫外可见光谱及元素分析。另外,复合物6a的结构通过X射线晶体学测定。紫外可见光谱研究表明,配体和金属配合物在365 nm紫外线照射下均表现出优异的芳基偶氮吡唑部分反式至顺式光异构化。化合物的顺式异构体可以在530 nm辐射下几乎定量地转换回更稳定的转化形式。金属离子的配位对配体的光开关性能没有显着影响。进行DFT和TD-DFT计算以优化配体的几何形状,并补充观察到的电子跃迁和吸收带的实验结果。从这些研究中获得的数据与实验结果非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号