当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Leuckart–Wallach Reaction in Flow for the Synthesis of Abemaciclib
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.oprd.7b00234 Michael O. Frederick , Mark A. Pietz , Douglas P. Kjell , Rachel N. Richey , Gregg A. Tharp , Taichiro Touge 1 , Naota Yokoyama 1 , Michio Kida 1 , Toshiyasu Matsuo 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.oprd.7b00234 Michael O. Frederick , Mark A. Pietz , Douglas P. Kjell , Rachel N. Richey , Gregg A. Tharp , Taichiro Touge 1 , Naota Yokoyama 1 , Michio Kida 1 , Toshiyasu Matsuo 1
Affiliation
|
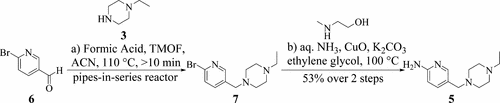
The development of a route for a key building block in the synthesis of abemaciclib is described. The route proceeds through a Leuckart–Wallach reductive amination in flow followed by an Ullmann amination with aqueous ammonia. Key to the Leuckart–Wallach reductive amination was the addition of trimethyl orthoformate for water removal, running the reaction continuously in a pipes-in-series reactor for rapid heat-up, and building a kinetic model to understand time and temperature parameters for the feed tank storage. The product of the Leuckart–Wallach reductive amination is forward processed in batch and telescoped with the Ullmann amination and subsequent workup. The development resulted in a robust process that has successfully been run on the production scale.
中文翻译:

用于合成Abemaciclib的流动Leuckart-Wallach反应的开发
描述了abemaciclib合成中关键构建模块的路线的开发。该路线通过流经Leuckart-Wallach的还原性胺化反应,然后进行氨水的Ullmann胺化反应。Leuckart-Wallach还原胺化的关键是添加原甲酸三甲酯以去除水分,在串联管式反应器中连续进行反应以快速加热,并建立动力学模型以了解进料的时间和温度参数储罐。Leuckart–Wallach还原胺化的产品分批进行正向加工,并通过Ullmann胺化进行伸缩和后续处理。这项开发产生了可靠的过程,该过程已成功地在生产规模上运行。
更新日期:2017-08-29
中文翻译:

用于合成Abemaciclib的流动Leuckart-Wallach反应的开发
描述了abemaciclib合成中关键构建模块的路线的开发。该路线通过流经Leuckart-Wallach的还原性胺化反应,然后进行氨水的Ullmann胺化反应。Leuckart-Wallach还原胺化的关键是添加原甲酸三甲酯以去除水分,在串联管式反应器中连续进行反应以快速加热,并建立动力学模型以了解进料的时间和温度参数储罐。Leuckart–Wallach还原胺化的产品分批进行正向加工,并通过Ullmann胺化进行伸缩和后续处理。这项开发产生了可靠的过程,该过程已成功地在生产规模上运行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号